Abstract
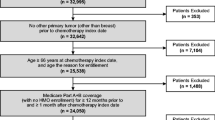
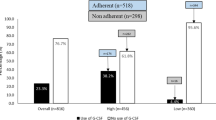
Effective systemic therapy is vital for successful breast cancer treatment, but early onset toxicities like neutropenia hinder systemic therapy administration, especially in the elderly. Primary prophylactic use of granulocyte-colony stimulating factors (G-CSF) helps prevent neutropenia, and according to some clinical trials, facilitates chemotherapy completion. Nevertheless, evidence supporting the effectiveness of primary prophylactic G-CSF in the elderly is limited. Thus, the ASCO recommendations for primary prophylactic G-CSF use in the elderly are not explicit. This retrospective observational study examined the association between primary prophylactic G-CSF administration at the start of first course chemotherapy with adequate first course chemotherapy and radiation therapy administration in elderly breast cancer patients. The study analyzes newly diagnosed breast cancer patients receiving chemotherapy present in the SEER-Medicare data from 1994 to 2003. To account for the non-random nature of the observational data, a non-parametric matching technique was used to pre-process the data before estimating the effect of primary prophylactic G-CSF on adequate chemotherapy and radiation therapy administration. Adequate chemotherapy was defined as administration of six or more cycles during the first course. Primary prophylactic G-CSF administered at the start of the first course chemotherapy was associated with a statistically significant increase in the probability of administration of six or more first course chemotherapy cycles by 29% [95% CI 7.7–50.6%] and any radiation therapy administration by 42% [95% CI 25.2–58.4%]. Primary prophylactic G-CSF use with the first course of chemotherapy is associated with improved chemotherapy completion rates and radiation therapy. These findings emphasize the clinical value of primary prophylactic G-CSF use for systemic therapy completion, and have implications for ASCO guidelines and medicare coverage policies.
Similar content being viewed by others
References
American Cancer Society (2009) Breast cancer facts and figures 2009–2010. American Cancer Society Inc, Atlanta
Earle CC, Nattinger AB, Potosky AL et al (2002) Identifying cancer relapse using SEER-medicare data. Med Care 40(Suppl 8):104–117
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomized trials. Lancet 365:1687–1717
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (1998) Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 352:930–942
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (1992) Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomised trials among 28,896 women. N Engl J Med 319:1681–1692
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (1988) Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 339:1–15, 71–85
Fisher B (1999) Highlights from recent national surgical adjuvant breast and bowel project studies in the treatment and prevention of breast cancer. CA Cancer J Clin 49:159–177
Fisher B, Redmond C, Legault PS et al (1990) Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the national surgical adjuvant breast and bowel project B-16. J Clin Oncol 8(6):1005–1018
Hortobagyi GN (1998) Treatment of breast cancer. N Engl J Med 339:974–984
Shayne M, Crawford J, Dale DC et al (2006) Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat 100:255–262
Ziegler J, Citron M (2006) Dose-dense adjuvant chemotherapy for breast cancer. Cancer Nurs 29(4):266–272
Chrischilles EA, Link KB, Scott SD et al (2003) Factors associated with early termination of CHOP therapy and the impact on survival among patients with chemosensitive intermediate grade non-Hodgkin’s lymphoma. Cancer Control 10(5):396–403
Hershman D, Neugut A, Jacobson JS et al (2007) Acute myeloid leukemia or mylodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 99(3):196–205
Weycker D, Hackett J, Edelsberg JS et al (2006) Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother 40:402–407
Ozer H, Armitage JO, Bennett CL (2000) Update of recommendations for the use of hematopoietic colony-stimulating factors; evidence-based, clinical practice guidelines. American society of clinical oncology growth factors expert panel. J Clin Oncol 19:3558–3585
Baquiran DC (2001) Biologic response modifiers. In: Baquiran DC (ed) Lippincott’s cancer chemotherapy handbook, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 13–41
Webster J, Lyman G (1996) Use of G-CSF to sustain dose intensity in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Control 3(6):519–523
Welte K, Gabrilove J, Bronchud M (1996) Filgrastim (r-metHuG-CSF): the first 10 years. Blood 88(6):1907–1929
Hryniuk WM (1988) The importance of dose intensity in the outcome of chemotherapy. In: DeVita V Jr, Hellman S, Rosenberg SA (eds) Important advances in oncology. J.B. Lippincott, Philadelphia, pp 121–141
Budman DR, Berry DA, Cirrincione CT (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The cancer and leukemia group B. J Natl Cancer Inst 90(16):1205–1211
Lyman GH, Kuderer N, Agboola O et al (2003) Evidence-based use of colony-stimulating factors in elderly cancer patients. Cancer Control 10(6):487–499
Goodwin JS, Samet JM, Hunt WC (1996) Determinants of survival in older cancer patients. J Natl Cancer Inst 88:1031–1038
Lee-Feldstein A, Anton-Culver H, Feldstein PJ (1994) Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. J Am Med Assoc 271:1163–1168
Early Breast Cancer Trialists’ Collaborative Group (1995) Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med 333:1444–1455
Fowble B, Goodman RL, Glick JH (1991) Breast cancer treatment: a comprehensive guide to management. Mosby Year Book, St. Louis
Welte K, Bonilla MA, Gabrilove JL et al (1987) Recombinant human granulocyte-colony stimulating factor: in vitro and in vivo effects on myelopoiesis. Blood Cells 13(1–2):17–30
Marks LB, Friedman HS, Kurtzberg J et al (1992) Reversal of radiation-induced neutropenia by granulocyte colony-stimulating factor. Med Pediatr Oncol 20(3):240–242
MacVittie TJ (1997) Therapy of radiation injury. Stem Cells 15(Suppl 2):263–268
Kuderer NN, Dale DC, Crawford J et al (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25(21):3158–3167
Hassett MJ, O’Malley AJ, Pakes JR (2006) Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98(16):1108–1117
Smith TJ, Khatcheressian J, Lyman GH et al (2006) Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205
Lyman GH, Dale DC, Crawford J (2003) Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 21:4524–4531
Package insert (2009) Neupogen (Filgrastim). Amgen. http://www.neupogen.com. Accessed 26 March 2009
Chrischilles EA, Rubenstein LM, Voelker MD, Wright et al. (2003) Granulocyte colony-stimulating factor use during first course chemotherapy for non-Hodgkin’s lymphoma: National SEER-medicare study. Session type: Poster session 929-I
Weycker D, Hackett J, Edelsberg JS et al. (2004) Duration of G-CSF therapy and risk of hospitalization for neutropenia or infection. ASCO Annual Meeting Proceedings (post-meeting edition). J Clin Oncol 22(14S): 6731
Scott SD, Chrischilles EA, Link BK et al (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin’s lymphoma treated with chemotherapy. J Manag Care Pharm 9(Suppl 2):15–21
Warren JL, Klabunde CN, Deborah Schrag et al (2002) Overview of the SEER-medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40(Suppl 8):3–18
Perry MC, Yarbro JW (1984) Toxicity of chemotherapy. Gruene & Stratton, Orlando
Shapiro CL, Recht A (2001) Side effects of adjuvant treatment of breast cancer. N Engl J Med 344(26):1997–2008
Crawford J, Kreisman H, Garewal H et al (1997) The impact of Filgrastim schedule variation on hematopoietic recovery post-chemotherapy. Ann Oncol 8:1117–1124
Chrischilles EA, Delgado DJ, Stolshek BS (2002) Impact of age and colony-stimulating factor use on hospital length of stay for febrile neutropenia in chop-treated non-Hodgkin’s lymphoma. Cancer Control 9(3):203–211
Gomez H, Hidalgo M, Casanova L et al (1998) Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: results of a multivariate analysis. J Clin Oncol 16:2065–2069
Du XL, Lairson DR, Begley E et al (2005) Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol 23(34):8620–8628
Lyman GH (2003) Risk assessment in oncology clinical practice: from risk factors to risk models. Oncology 17(11):8–13
Lyman GH, Kuderer NM (2004) Economics of hematopoietic growth factors. In: Morstyn G, Foote M, Lieschke GJ (eds) Hematopoietic growth factors in oncology: basic science and clinical therapeutics. Humana Press Inc, Totowa, pp 409–443
Rajan S, Lyman G, Carpenter W, Stearns S (2010) Chemotherapy characteristics are important predictors of primary prophylactic CSF administration in older patients with breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-010-1216-1
Ho DE, Imai IK, King G et al (2007) Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Pol Anal 15:199–236
King G, Zeng L (2006) The dangers of extreme counterfactuals. Pol Anal 14:131–159
Robins J, Rotnitzky A (2001) Comment on “Inference for semiparametric models: some questions and an answer” by Bickel and Kwon. Statisica Sinica 11:920–936
Sekhon JS, Mebane WR (1998) Genetic optimization using derivatives. Pol Anal 7(1):187–210
Diamond A, Sekhon J (2008) Genetic matching for estimating causal effects: a general multivariate matching method for achieving balance in observational studies. Institute of Government Studies (e-paper); Paper WP2006-35
Ardizzoni A, Venturini M, Sertoli MR et al (1994) Granulocyte-macrophage colony-stimulating factor (GM-CSF) allows acceleration and dose intensity increase of CEF chemotherapy: a randomised study in patients with advanced breast cancer. Br J Cancer 69(2):385–391
Engelhard M, Gerhartz H, Brittinger G et al (1994) Cytokine efficiency in the treatment of high-grade malignant non-Hodgkin’s lymphomas: results of a randomized double-blind placebo-controlled study with intensified COP-BLAM/- rhGM-CSF. Ann Oncol 5(Suppl 2):123–125
Fridrik MA, Greil R, Hausmaninger H et al (1997) Randomized open label phase III trial of CEOP/IMVP-Dexa alternating chemotherapy and filgrastim versus CEOP/IMVP-Dexa alternating chemotherapy for aggressive non-Hodgkin’s lymphoma (NHL). A multicenter trial by the Austrian working group for medical tumor therapy. Ann Hemtol 75:135–140
Fukuoka M, Masuda N, Negoro S et al (1997) CODE chemotherapy with and without granulocyte colony-stimulating factor in small-cell lung cancer. Br J Cancer 75:306–309
Heil G, Hoelzer D, Sanz MA et al (1997) A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The international acute myeloid leukemia study group. Blood 90(12):4710–4718
Hidalgo M, Mendiola C, Lo′pez-Vega JM et al (1998) A multicenter randomized Phase II trial of granulocytecolony stimulating factor-supported, platinum-based chemotherapy with flexible midcycle cisplatin administration in patients with advanced ovarian carcinoma. PSAMOMA cooperative group, Spain. Cancer 83:719–725
Jost LM, Pichert G, Stahel RA (1990) Placebo controlled phase I/II study of subcutaneous GM-CSF in patients with germ cell tumors undergoing chemotherapy. Ann Oncol 1:439–442
Kaplan LD, Kahn JO, Crowe S et al (1991) Clinical and virologic effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients receiving chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma: results of a randomized trial. J Clin Oncol 9:929–940
Kotake T, Miki T, Akaza H et al (1991) Effect of recombinant granulocyte colony-stimulating factor (rG-CSF) on chemotherapy-induced neutropenia in patients with urogenital cancer. Cancer Chemother Pharmacol 27:253–257
Miles DW, Fogarty O, Ash CM et al (1994) Received dose-intensity: a randomized trial of weekly chemotherapy with and without granulocyte colony-stimulating factor in small-cell lung cancer. J Clin Oncol 12(1):77–82
Hansen F, Stenbygaard L, Skovsgaard T (1995) Effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on hematologic toxicity induced by highdose chemotherapy in patients with metastatic breast cancer. Acta Oncol 34:919–924
Jones SE, Schottstaedt MW, Duncan LA et al (1996) Randomized double-blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate-dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. J Clin Oncol 14:2976–2978
Kuderer NM, Crawford J, Dale DC (2005) Meta-analysis of prophylactic granulocyte colony-stimulating factor (G-CSF) in cancer patients receiving chemotherapy (ASCO Annual Meeting Proceedings). J Clin Oncol 23(16S): 8117
Lyman GH, Kuderer NM, Djulbegovic B (2002) Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med 112:406–411
Pfreundschuh M, Hasenclever D, Loeffler M (2001) German Hodgkin’s lymphoma study group. Dose escalation of cytotoxic drugs using haematopoietic growth factors: a randomized trial to determine the magnitude of increase provided by GM-CSF. Ann Oncol 12:471–477
Steward WP, von Pawel J, Gatzemeier U (1998) Effects of granulocyte-macrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 16:642–650
Stöger H, Samonigg H, Krainer M (1998) Dose intensification of epidoxorubicin and cyclophosphamide in metastatic breast cancer: a randomised study with two schedules of granulocyte-macrophage colony stimulating factor. Eur J Cancer 34(4):482–488
Woll PJ, Hodgetts J, Lomax L (1995) Can cytotoxic dose-intensity be increased by using granulocyte colony-stimulating factor? A randomized controlled trial of lenograstim in small-cell lung cancer. J Clin Oncol 13:652–659
Zagonel V, Babare R, Merola MC (1994) Cost-benefit of granulocyte colony-stimulating factor administration in older patients with non-Hodgkin’s lymphoma treated with combination chemotherapy. Ann Oncol 5(Suppl 2):127–132
Shayne M, Culakova E, Poniewierski MS et al (2007) Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 110(7):1611–1620
Rajan S, Lyman G, Stearns S, Carpenter W (2011) Effect of primary prophylactic G-CSF use on incidence of neutropenia hospitalizations for elderly early-stage breast cancer patients receiving chemotherapy. Med Care. Accessed 29 January 2011
Acknowledgments
This study used the linked SEER-medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the surveillance, epidemiology, and end results (SEER) Program tumor registries in the creation of the SEER-medicare database. We sincerely appreciate the support provided by Paul Godley for his interest in this topic and providing us with data access and useful advice. We also, appreciate George Pink and Morris Weinberger for their expert opinions and advice as part of the first author’s Doctoral Thesis Committee.
Conflict of interest
This study was not funded by any drug company or organization with stakes or financial incentives associated with the drugs analyzed in this study. The first author was a graduate student at the time of the study and was partly supported by a research assistant position at UNC-Chapel Hill from a Department of Defense grant for a prostate cancer study unrelated to the study presented in this article. This study was completed as part of the first author’s Ph.D. dissertation. One of the authors, Dr. Gary Lyman, is a principal investigator on a research grant to Duke University from Amgen, Inc for work completely unrelated with this study or the dissertation. Dr. Lyman is one of the most experienced clinical investigators in this field and thus provided expert advice as a member of the dissertation committee. The results presented in this publication were presented, in part, at the 2010 Annual Research Meeting of Academy Health in Boston, MA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rajan, S.S., Stearns, S.C., Lyman, G.H. et al. Effect of primary prophylactic G-CSF use on systemic therapy administration for elderly breast cancer patients. Breast Cancer Res Treat 130, 255–266 (2011). https://doi.org/10.1007/s10549-011-1553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1553-8