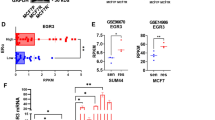
Abstract
Overexpression and altered function of EphA2 receptor tyrosine kinase are critical in the progression of breast cancer and provide a target for breast cancer therapy. We have previously demonstrated that EphA2 overexpression decreases estrogen dependence and Tamoxifen sensitivity both in vitro and in vivo. EA5, a novel monoclonal antibody that mimicks the binding of ephrin A to EphA2, reverses the effect of EphA2 overexpression and restores Tamoxifen sensitivity in EphA2-transfected MCF-7 cells in vitro. To explore the role of EphA2 overexpression on ER-dependent mechanisms, we used two different ER+/EphA2-transfected cell line models (MCF-7neo/MCF-7EphA2 and T47Dneo/T47DEphA2). EA5 inhibits primary tumor growth and restores Tamoxifen sensitivity in the MCF-7EphA2 xenografts. Using the T47DEphA2 in vitro model, we verified that EphA2 decreases ER activation in response to E2 stimulation consistent with our earlier results in MCF-7EphA2 model. We found no direct interaction between ER and EphA2 and no difference in expression of canonical ER-dependent proteins or ER co-regulators. However, E2 stimulation phosphorylates FAKTyr925 only in ER+/EphA2+ cell lines. Treatment of T47DEphA2 cells with EA5 and Tamoxifen leads to dephosphorylation of FAKTyr925 in response to E2. Our data demonstrate that dual targeting of EphA2 and ER is a promising approach for delaying resistance to Tamoxifen. The data support our hypothesis that EphA2 impacts ER function via a FAK dependent pathway.






Similar content being viewed by others
References
Eph Nomenclature Committee (1997) Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 90:403–404
Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133:38–52
Vaught D, Brantley-Sieders DM, Chen J (2008) Eph receptors in breast cancer: roles in tumor promotion and tumor suppression. Breast Cancer Res 10:217
Lindberg RA, Hunter T (1990) cDNA cloning, characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol 10:6316–6324
Wykosky J, Debinski W (2008) The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 6:1795–1806
Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS (2001) EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res 61:2301–2306
Zantek ND, Walker-Daniels J, Stewart J, Hansen RK, Robinson D, Miao H, Wang B, Kung HJ, Bissell MJ, Kinch MS (2001) MCF-10A-NeoST: a new cell system for studying cell-ECM and cell-cell interactions in breast cancer. Clin Cancer Res 7:3640–3648
Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS (2002) Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem 85:714–720
Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS (1999) E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ 10:629–638
Pan M (2005) Overexpression of EphA2 gene in invasive human breast cancer and its association with hormone receptor status [ASCO annual meeting proceedings]. J Clin Oncol 23:9583
Martin KJ, Patrick DR, Bissell MJ, Fournier MV (2008) Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One 3:e2994
Fournier MV, Martin KJ, Kenny PA et al (2006) Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res 66:7095–7102
Fox BP, Kandpal RP (2004) Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun 318:882–892
Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, Chen J (2008) The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Investig 118:64–78
Lu M, Miller KD, Gökmen-Polar Y, Jeng MH, Kinch MS (2003) EphA2 overexpression decreases estrogen dependence and tamoxifen sensitivity. Cancer Res 63:3425–3429
Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS (2002) Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res 62:2840–2847
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121
Gökmen-Polar Y, Mehta R, Tuzmen S, Mousses S, Thorat MA, Sanders KL, Turbin D, Leung S, Huntsman DG, Sledge GW Jr, Badve S (2010) Differential subcellular expression of protein kinase C betaII in breast cancer: correlation with breast cancer subtypes. Breast Cancer Res Treat. doi:10.1007/s10549-010-0733-2
Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P (2009) EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res 69:2072–2081
McKenna NJ, O’Malley BW (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474
Hall JM, McDonnell DP (2005) Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv 5:343–357
Schiff R, Massarweh S, Shou J, Osborne CK (2003) Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 9:447S–454S
Katoh M (2007) WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13:4042–4045
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
van Amerongen R, Mikels A, Nusse R (2008) Alternative Wnt signaling is initiated by distinct receptors. Sci Signal 1:re9
Neth P, Ries C, Karow M, Egea V, Ilmer M, Jochum M (2007) The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev 3:18–29
Carter N, Nakamoto T, Hirai H, Hunter T (2002) EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat Cell Biol 4:565–573
Miao H, Burnett E, Kinch M, Simon E, Wang B (2000) Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol 2:62–69
Schlaepfer DD, Mitra SK, Ilic D (2004) Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta 1692:77–102
Kohn AD, Moon RT (2005) Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38:439–446
Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD (2003) Differential regulation of cell motility and invasion by FAK. J Cell Biol 160:753–767
Kohno M, Hasegawa H, Miyake M, Yamamoto T, Fujita S (2002) CD151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase. Int J Cancer 97:336–343
Kinch MS, Carles-Kinch K (2003) Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis 20:59–68
Dodelet VC, Pasquale EB (2000) Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene 19:5614–5619
Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA, Kinch MS (2003) Differential EphA2 epitope display on normal versus malignant cells. Cancer Res 63:7907–7912
Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK (2004) Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10:331S–336S
Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, Huang TH, Nephew KP (2006) Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res 66:11954–11966
Katoh Y, Katoh M (2006) Comparative integromics on Ephrin family. Oncol Rep 15:1391–1395
Wang Y (2009) Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther 8:2103–2109
Schlessinger K, Hall A, Tolwinski N (2009) Wnt signaling pathways meet Rho GTPases. Genes Dev 23:265–277
Acknowledgments
We thank Dr. D. Knapp (Purdue University) for EphA2 constructs; Dr. M-H Jeng (Indiana University) for ERE-luciferase, TK-luciferase, purified GST, GST-ER alpha, SRC-1 and AIB1 plasmids; Dr. H. Nakshatri (Indiana University) for NCoR1 and SMRT plasmids. We also thank Karen Coffman (MedImmune) for her assistance in providing EA5 antibody. Gene array was by Miltenyi Biotech Inc. Microarray Service. This work was supported by Susan G. Komen grant (BCTR0503531) to K.D. Miller.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gökmen-Polar, Y., Toroni, R.A., Hocevar, B.A. et al. Dual targeting of EphA2 and ER restores tamoxifen sensitivity in ER/EphA2-positive breast cancer. Breast Cancer Res Treat 127, 375–384 (2011). https://doi.org/10.1007/s10549-010-1004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1004-y