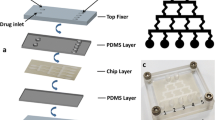
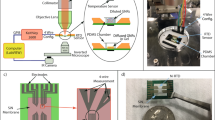
Abstract
Temperature is a critical extrinsic physical parameter that determines cell fate. Hyperthermia therapy has become an efficient treatment for tumor ablation. To understand the response of tumor cells under thermal shocks, we present a paper-based photothermal array that can be conveniently coupled with commercial 96-well cell culture plates. This paper chip device was fabricated in one step using Parafilm® and Kimwipers® based on a heat lamination strategy. Liquid was completely adsorbed and confined within the cellulose fibres of hydrophilic regions. Then, Prussian blue nanoparticles (PB NPs) as the photothermal initiator were introduced into the loading wells, and thermal energy was generated via near infrared (NIR) laser irradiation. After assembling the paper device with a 96-well plate, the temperature of each well could be individually controlled by varying the loading amount of PB NPs and laser irradiation time. As a proof-of-concept study, the effects of local thermal shocks on HeLa cells were investigated using MTT cell viability assay and Live/Dead cell staining. The variation of cell viability could be monitored in situ with controllable temperature elevation. The proposed paper photothermal array loaded with thermal initiators represents an enabling tool for investigating the hyperthermia responses of biological cells. Moreover, the facile fabrication technique for paper patterning is advantageous for customizing high-throughput microfluidic paper-based analytical devices (μPADs) with extremely low cost.






Similar content being viewed by others
References
K. Abe, K. Suzuki, D. Citterio, Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 80, 6928–6934 (2008)
G. Andocs, M.U. Rehman, Q.L. Zhao, Y. Tabuchi, M. Kanamori, T. Kondo, Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discovery 2, 16039 (2016)
Z.H. Bao, X.R. Liu, Y.D. Liu, H.Z. Liu, K. Zhao, Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 11, 349–364 (2016)
K.W. Baumann, J.M. Baust, K.K. Snyder, J.G. Baust, R.G. Van Buskirk, Characterization of pancreatic cancer cell thermal response to heat ablation or Cryoablation. Technol. Cancer Res. Treat. 16, 393–405 (2017)
H.M. Beere, "The stress of dying": The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117, 2641–2651 (2004)
A. Bettaieb, D.A. Averill-Bates, Thermotolerance induced at a mild temperature of 40°C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochim. Biophys. Acta, Mol. Cell Res. 1853, 52–62 (2015)
C. Brace, Thermal tumor ablation in clinical use. IEEE Pulse 2, 28–38 (2011)
S. Chatterjee, T.F. Burns, Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 18, 1978 (2017)
E.A. Craig, B.D. Gambill, R.J. Nelson, Heat-shock proteins - molecular chaperones of protein biogenesis. Microbiol. Rev. 57, 402–414 (1993)
M.J. Cziesielski, Y.J. Liew, G.X. Cui, S. Schmidt-Roach, S. Campana, C. Marondedze, M. Aranda, Multi-omics analysis of thermal stress response in a zooxanthellate cnidarian reveals the importance of associating with thermotolerant symbionts. Proc. R. Soc. B 285, 20172654 (2018)
R.D. Deegan, O. Bakajin, T.F. Dupont, G. Huber, S.R. Nagel, T.A. Witten, Capillary flow as the cause of ring stains from dried liquid drops. Nature 389, 827 (1997)
W. Dungchai, O. Chailapakul, C.S. Henry, A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 136, 77–82 (2011)
X.E. Fang, S.S. Wei, J.L. Kong, Paper-based microfluidics with high resolution, cut on a glass fiber membrane for bioassays. Lab Chip 14, 911–915 (2014)
G.L. Fu, W. Liu, S.S. Feng, X.L. Yue, Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem. Commun. 48, 11567–11579 (2012)
G.L. Fu, W. Liu, Y.Y. Li, Y.S. Jin, L.D. Jiang, X.L. Liang, S.S. Feng, Z.F. Dai, Magnetic Prussian blue nanoparticles for targeted photothermal therapy under magnetic resonance imaging guidance. Bioconjug. Chem. 25, 1655–1663 (2014)
Y. He, W.B. Wu, J.Z. Fu, Rapid fabrication of paper-based microfluidic analytical devices with desktop stereolithography 3D printer. RSC Adv. 5, 2694–2701 (2015a)
Y. He, Y. Wu, J.Z. Fu, W.B. Wu, Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv. 5, 78109–78127 (2015b)
H.C. Huang, K. Rege, J.J. Heys, Spatiotemporal temperature distribution and Cancer cell death in response to extracellular hyperthermia induced by gold Nanorods. ACS Nano 4, 2892–2900 (2010)
M. Hussong, C. Kaehler, M. Kerick, C. Grimm, A. Franz, B. Timmermann, F. Welzel, J. Isensee, T. Hucho, S. Krobitsch, M.R. Schweiger, The bromodomain protein BRD4 regulates splicing during heat shock. Nucleic Acids Res. 45, 382–394 (2017)
D. Jaque, L.M. Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J.L. Plaza, E. Martín Rodrígueza, J. García Soléa, Nanoparticles for photothermal therapies. Nanoscale 6, 9494–9530 (2014)
H.H. Kampinga, J.F. Brunsting, G.J.J. Stege, P.W.J.J. Burgman, A.W.T. Konings, Thermal protein denaturation and protein aggregation in cells made Thermotolerant by various chemicals - role of heat-shock proteins. Exp. Cell Res. 219, 536–546 (1995)
B. Kong, J.H. Seog, L.M. Graham, S.B. Lee, Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine (London, U. K.) 6, 929–941 (2011)
J.R. Lepock, Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 19, 252–266 (2003)
X. Li, D.R. Ballerini, W. Shen, A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 6, 11301–1130113 (2012)
P. Lisowski, P.K. Zarzycki, Microfluidic paper-based analytical devices (muPADs) and micro total analysis systems (muTAS): Development, applications and future trends. Chromatographia 76, 1201–1214 (2013)
X.J. Liu, B. Li, F.F. Fu, K.B. Xu, R.J. Zou, Q. Wang, B. Zhang, Z. Chen, J. Hu, Facile synthesis of biocompatible cysteine-coated CuS nanoparticles with high photothermal conversion efficiency for cancer therapy. Dalton Trans. 43, 11709–11715 (2014)
A.W. Martinez, S.T. Phillips, B.J. Wiley, M. Gupta, G.M. Whitesides, FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 8, 2146–2150 (2008)
R.I. Morimoto, M.P. Kline, D.N. Bimston, J.J. Cotto, The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 32, 17–29 (1997)
T. Nagaya, Y. Nakamura, K. Sato, T. Harada, P.L. Choyke, H. Kobayashi, Near infrared photoimmunotherapy of B-cell lymphoma. Mol. Oncol. 10, 1404–1414 (2016)
R. Ota, K. Yamada, K. Suzuki, D. Citterio, Quantitative evaluation of analyte transport on microfluidic paper-based analytical devices (μPADs). Analyst 143, 643–653 (2018)
K. Richter, M. Haslbeck, J. Buchner, The heat shock response: Life on the verge of death. Mol. Cell 40, 253–266 (2010)
A. Samali, C.I. Holmberg, L. Sistonen, S. Orrenius, Thermotolerance and cell death are distinct cellular responses to stress: Dependence on heat shock proteins. FEBS Lett. 461, 306–310 (1999)
Y. Sameenoi, P.N. Nongkai, S. Nouanthavong, C.S. Henry, D. Nacapricha, One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 139, 6580–6588 (2014)
M. Sher, R. Zhuang, U. Demirci, W. Asghar, Paper-based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert. Rev. Mol. Diagn. 17, 351–366 (2017)
M. Shokouhimehr, E.S. Soehnlen, J.H. Hao, M. Griswold, C. Flask, X.D. Fan, J.P. Basilion, S. Basu, S.P.D. Huang, Dual purpose Prussian blue nanoparticles for cellular imaging and drug delivery: A new generation of T-1-weighted MRI contrast and small molecule delivery agents. J. Mater. Chem. 20, 5251–5259 (2010)
N.M. Templeman, S. LeBlanc, S.F. Perry, S. Currie, Linking physiological and cellular responses to thermal stress: Beta-adrenergic blockade reduces the heat shock response in fish. J. Comp. Physiol. B. 184, 719–728 (2014)
P. Xue, J.N. Bao, Y.F. Wu, Y.L. Zhang, Y.J. Kang, Magnetic Prussian blue nanoparticles for combined enzyme-responsive drug release and photothermal therapy. RSC Adv. 5, 28401–28409 (2015)
P. Xue, L.H. Sun, Q. Li, L. Zhang, Z.G. Xu, C.M. Li, Y.J. Kang, PEGylated magnetic Prussian blue nanoparticles as a multifunctional therapeutic agent for combined targeted photothermal ablation and pH triggered chemotherapy of tumour cells. J. Colloid Interface Sci. 509, 384–394 (2018)
Y. Yang, E. Noviana, M.P. Nguyen, B.J. Geiss, D.S. Dandy, C.S. Henry, Paper-based microfluidic devices: Emerging themes and applications. Anal. Chem. 89, 71–91 (2017)
A.K. Yetisen, M.S. Akram, C.R. Lowe, Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 13, 2210–2251 (2013)
L.L. Zou, H. Wang, B. He, L.J. Zeng, T. Tan, H.Q. Cao, et al., Current approaches of photothermal therapy in treating cancer metastasis with Nanotherapeutics. Theranostics 6, 762–772 (2016)
Acknowledgements
P. X., L. Z. and Y. K. acknowledge the Fundamental Research Funds for Central Universities (XDJK2018C010, XDJK2017C001, XDJK2016A010 and SWU115059), a start-up grant from Southwest University (SWU116032) and National Natural Science Foundation of China (51703186, 31671037).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 273 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Sun, L., Hou, M. et al. A paper-based photothermal array using Parafilm to analyze hyperthermia response of tumour cells under local gradient temperature. Biomed Microdevices 20, 68 (2018). https://doi.org/10.1007/s10544-018-0311-7
Published:
DOI: https://doi.org/10.1007/s10544-018-0311-7