Abstract
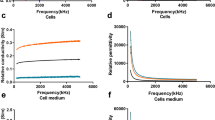
Triple negative breast cancer (TNBC) is highly aggressive and has a poor prognosis when compared to other molecular subtypes. In particular, the claudin-low subtype of TNBC exhibits tumor-initiating/cancer stem cell like properties. Here, we seek to find new biomarkers to discriminate different forms of TNBC by characterizing their bioimpedance. A customized bioimpedance sensor with four identical branched microelectrodes with branch widths adjusted to accommodate spreading of individual cells was fabricated on silicon and pyrex/glass substrates. Cell analyses were performed on the silicon devices which showed somewhat improved inter-electrode and intra-device reliability. We performed detailed analysis of the bioimpedance spectra of four TNBC cell lines, comparing the peak magnitude, peak frequency and peak phase angle between claudin-low TNBC subtype represented by MDA-MB-231 and Hs578T with that of two basal cells types, the TNBC MDA-MB-468, and an immortalized non-malignant basal breast cell line, MCF-10A. The claudin-low TNBC cell lines showed significantly higher peak frequencies and peak phase angles than the properties might be useful in distinguishing the clinically significant claudin-low subtype of TNBC.






Similar content being viewed by others
References
M. Abdolahad, M. Janmaleki, M. Taghinejad, H. Taghnejad, F. Salehi, S. Mohajerzadeh, Single-cell resolution diagnosis of cancer cells by carbon nanotube electrical spectroscopy. Nanoscale 5, 3421–3427 (2013)
F. Alexander Jr, D.T. Price, S. Bhansali, Optimization of interdigitated electrode (IDE) arrays for impedance based evaluation of Hs 578T cancer cells. Journal of Physics: Conference Series: IOP Publishing, (2010), p. 012134
S.K. Arya, K.C. Lee, D. Bin Dah’alan, Daniel, A.R.A. Rahman, Breast tumor cell detection at single cell resolution using an electrochemical impedance technique. Lab. Chip. 12, 2362–8 (2012)
F. Asphahani, M. Thein, O. Veiseh, D. Edmondson, R. Kosai, M. Veiseh et al., Influence of cell adhesion and spreading on impedance characteristics of cell-based sensors. Biosens. Bioelectron. 23, 1307–1313 (2008)
P. Carotenuto, C. Roma, A.M. Rachiglio, G. Botti, A. D’Alessio, N. Normanno, Triple negative breast cancer: from molecular portrait to therapeutic intervention. Crit. Rev. Eukaryot. Gene Expr. 20, 17–34 (2010)
N. Chauveau, L. Hamzaoui, P. Rochaix, B. Rigaud, J.J. Voigt, J.P. Morucci, Ex vivo discrimination between normal and pathological tissues in human breast surgical biopsies using bioimpedance spectroscopy. Electr. Bioimpedance Methods Appl. Med. Biotechnol. 873, 42–50 (1999)
K.J. Chavez, S.V. Garimella, S. Lipkowitz, Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 32, 35–48 (2011)
L. Chin, J.N. Andersen, P.A. Futreal, Cancer genomics: from discovery science to personalized medicine. Nat. Med. 17, 297–303 (2011)
Y. Cho, H.S. Kim, A.B... Frazier, Z.G. Chen, D.M. Shin, A. Han, Whole-cell impedance analysis for highly and poorly metastatic cancer cells. J. Microelectromech. Syst. 18, 808–817 (2009)
J. Choi, W.H. Jung, J.S. Koo, Clinicopathologic features of molecular subtypes of triple negative breast cancer based on immunohistochemical markers. Histol. Histopathol. 27, 1481–1493 (2012)
B. Cornish, M. Chapman, C. Hirst, B. Mirolo, I. Bunce, L. Ward et al., Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 34, 2–11 (2001)
C.J. Creighton, X.X. Li, M. Landis, J.M. Dixon, V.M. Neumeister, A. Sjolund et al., Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. U. S. A. 106, 13820–13825 (2009)
F. Di Cello, L. Cope, H.L. Li, J. Jeschke, W. Wang, S.B. Baylin et al., Methylation of the claudin 1 promoter is associated with loss of expression in estrogen receptor positive breast cancer. PLoS ONE 8, 8 (2013)
V.C. Fogg, C.J. Liu, B. Margolis, Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J. Cell Sci. 118, 2859–2869 (2005)
R. Gerhard, S. Ricardo, A. Albergaria, M. Gomes, A.R. Silva, A.F. Logullo et al., Immunohistochemical features of claudin-low intrinsic subtype in metaplastic breast carcinomas. Breast 21, 354–360 (2012)
I. Giaever, C.R. Keese, A morphological biosensor for mammalian-cells. Nature 366, 591–592 (1993)
W.D. Gregory, J.J. Marx, C.W. Gregory, W.M. Mikkelson, J.A. Tjoe, J. Shell, The cole relaxation frequency as a parameter to identify cancer in breast tissue. Med. Phys. 39, 4167–4174 (2012)
I.R. Gupta, A.K. Ryan, Claudins: unlocking the code to tight junction function during embryogenesis and in disease. Clin. Genet. 77, 314–325 (2010)
A. Han, L. Yang, A.B... Frazier, Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy. Clin. Cancer Res. 13, 139–143 (2007)
J.C. Harrell, A. Prat, J.S. Parker, C. Fan, X.P. He, L. Carey et al., Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res. Treat. 132, 523–535 (2012)
J.C. Harrell, A.D. Pfefferle, N. Zalles, A. Prat, C. Fan, A. Khramtsov, et al. Endothelial-like properties of claudin-low breast cancer cells promote tumor vascular permeability and metastasis. Clin. Exp. Metastasis (2013)
K. Heileman, J. Daoud, M. Tabrizian, Dielectric spectroscopy as a viable biosensing tool for cell and tissue characterization and analysis. Biosens. Bioelectron. 49, 348–359 (2013)
L.M. Heiser, A. Sadanandam, W.L. Kuo, S.C. Benz, T.C. Goldstein, S. Ng et al., Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 109, 2724–2729 (2012)
B.T. Hennessy, A.M. Gonzalez-Angulo, K. Stemke-Hale, M.Z. Gilcrease, S. Krishnamurthy, J.S. Lee et al., Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 69, 4116–4124 (2009)
J.I. Herschkowitz, W. Zhao, M. Zhang, J. Usary, G. Murrow, D. Edwards et al., Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proc. Natl. Acad. Sci. U. S. A. 109, 2778–2783 (2012)
J.L. Hong, K.C. Lan, L.S. Jang, Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sensors Actuators B-Chem. 173, 927–934 (2012)
X.Q. Huang, D. Nguyen, D.W. Greve, M.M. Domach, Simulation of microelectrode impedance changes due to cell growth. IEEE Sensors J. 4, 576–583 (2004)
C.A. Hudis, L. Gianni, Triple-negative breast cancer: an unmet medical need. Oncologist 16, 1–11 (2011)
H.-G. Jahnke, A. Heimann, R. Azendorf, K. Mpoukouvalas, O. Kempski, A.A. Robitzki et al., Impedance spectroscopy—an outstanding method for label-free and real-time discrimination between brain and tumor tissue in vivo. Biosens. Bioelectron. 46, 8–14 (2013)
C.M. Lo, C.R. Keese, I. Giaever, Impedance analysis of MDCK cells measured by electric cell-substrate impedance sensing. Biophys. J. 69, 2800–2807 (1995)
S.L. Lu, K. Singh, S. Mangray, R. Tavares, L. Noble, M.B. Resnick et al., Claudin expression in high-grade invasive ductal carcinoma of the breast: correlation with the molecular subtype. Mod. Pathol. 26, 485–495 (2013)
A. Marshall, V. Pai, M. Sartor, N. Horseman, In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 335, 383–395 (2009)
H. Masuda, K.A. Baggerly, Y. Wang, Y. Zhang, A.M. Gonzalez-Angulo, F. Meric-Bernstam, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. (2013)
T. Morimoto, S. Kimura, Y. Konishi, K. Komaki, T. Uyama, Y. Monden et al., A study of the electrical bio-impedance of tumors. Investig. Surg. 6, 25–32 (1993)
S. Narayanan, M. Nikkhah, J.S. Strobl, M. Agah, Analysis of the passivation layer by testing and modeling a cell impedance micro-sensor. Sensors Actuators A-Phys. 159, 241–247 (2010)
R.M. Neve, K. Chin, J. Fridlyand, J. Yeh, F.L. Baehner, T. Fevr et al., A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006)
E.Y.K. Ng, S.V. Sree, K.H. Ng, G. Kaw, The use of tissue electrical characteristics for breast cancer detection: a perspective review. Technol. Cancer Res. Treat. 7, 295–308 (2008)
H. Pick, S. Terrettaz, O. Baud, O. Laribi, C. Brisken, H. Vogel, Monitoring proliferative activities of hormone-like odorants in human breast cancer cells by gene transcription profiling and electrical impedance spectroscopy. Biosens. Bioelectron. 50, 431–436 (2013)
A. Prat, C.M. Perou, Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 5, 5–23 (2011)
A. Prat, J.S. Parker, O. Karginova, C. Fan, C. Livasy, J.I. Herschkowitz et al., Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12, 18 (2010)
D.T. Price, A.R.A. Rahman, S. Bhansali, Design rule for optimization of microelectrodes used in electric cell-substrate impedance sensing (ECIS). Biosens. Bioelectron. 24, 2071–2076 (2009)
G.F. Qiao, W. Wang, W. Duan, F. Zheng, A.J. Sinclair, C.R. Chatwin, Bioimpedance analysis for the characterization of breast cancer cells in suspension. IEEE Trans. Biomed. Eng. 59, 2321–2329 (2012)
E.A. Rakha, S. Chan, Metastatic triple-negative breast cancer. Clin. Oncol. 23, 587–600 (2011)
O. Raneta, V. Bella, L. Bellova, E. Zamecnikova, The use of electrical impedance tomography to the differential diagnosis of pathological mammographic/sonographic findings. Neoplasma 60, 647–654 (2013)
S. Ricardo, R. Gerhard, J.F. Cameselle-Teijeiro, F. Schmitt, J. Paredes, Claudin expression in breast cancer: high or low, what to expect? Histol. Histopathol. 27, 1283–1295 (2012)
A. Romero, A. Prat, J.A. García-Sáenz, N.del Prado, A. Pelayo, V. Furió, et al. Assignment of tumor subtype by genomic testing and pathologic-based approximations: implications on patient’s management and therapy selection. Clin. Transl. Oncol. (2013)
D. Sarrió, S. M. Rodriguez-Pinilla, D. Hardisson, A. Cano, G. Moreno-Bueno, J. Palacios, Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 68, 989–997 (2008)
D. Sarrio, J. Palacios, M. Hergueta-Redondo, G. Gomez-Lopez, A. Cano, G. Moreno-Bueno, Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 9, 14 (2009)
D. Sarrio, C.K. Franklin, A. Mackay, J.S. Reis, C.M. Isacke, Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells 30, 292–303 (2012)
C.L. Sommers, S.W. Byers, E.W. Thompson, J.A. Torri, E.P. Gelmann, Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res. Treat. 31, 325–335 (1994)
V. Srinivasaraghavan, J. Strobl, M. Agah, Chemical Induced Impedance Spectroscopy for Single Cancer Cell Detection (Solid-State Sensors, Actuators and Microsystems Conference (Transducers), Beijing, 2011a), pp. 2247–2250
V. Srinivasaraghavan, J. Strobl, M. Agah, Detection of Breast Cancer Cells in Tri-Culture Using Impedance Spectroscopy. 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS, Seattle, 2011b), pp. 1713–1715
V. Srinivasaraghavan, J. Strobl, M. Agah, Bioimpedance rise in response to histone deacetylase inhibitor is a marker of mammary cancer cells within a mixed culture of normal breast cells. Lab Chip 12, 5168–5179 (2012)
V. Srinivasaraghavan, J. Strobl, D. Wang, J.R. Heflin, M. Agah, A comparative study of nano-scale coatings on gold electrodes for bioimpedance studies of breast cancer cells. Biomed Microdevices 1–8 (2014)
J.S. Strobl, M. Nikkhah, M. Agah, Actions of the anti-cancer drug suberoylanilide hydroxamic acid (SAHA) on human breast cancer cytoarchitecture in silicon microstructures. Biomaterials 31, 7043–7050 (2010)
V. Walia, Y. Yu, D. Cao, M. Sun, J.R. McLean, B.G. Hollier et al., Loss of breast epithelial marker hCLCA2 promotes epithelial-to-mesenchymal transition and indicates higher risk of metastasis. Oncogene 31, 2237–2246 (2012)
J. Wegener, C.R. Keese, I. Giaever, Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 259, 158–166 (2000)
X.Y. Wu, H.X. Chen, B. Parker, E. Rubin, T. Zhu, J.S. Lee et al., HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 66, 9527–9534 (2006)
L.J. Yang, L.R. Arias, T.S. Lane, M.D. Yancey, J. Mamouni, Real-time electrical impedance-based measurement to distinguish oral cancer cells and non-cancer oral epithelial cells. Anal. Bioanal. Chem. 399, 1823–1833 (2011)
D.R. Youlden, S.M. Cramb, N.A.M. Dunn, J.M. Muller, C.M. Pyke, P.D. Baade, The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 36, 237–248 (2012)
Acknowledgments
We acknowledge the morphology service laboratory and Kathy Lowe for support with sample preparation for SEM imaging. This work was primarily supported by the National Science Foundation IDR Award: ECCS-0925945.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivasaraghavan, V., Strobl, J. & Agah, M. Microelectrode bioimpedance analysis distinguishes basal and claudin-low subtypes of triple negative breast cancer cells. Biomed Microdevices 17, 80 (2015). https://doi.org/10.1007/s10544-015-9977-2
Published:
DOI: https://doi.org/10.1007/s10544-015-9977-2