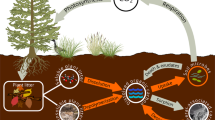
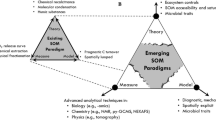
Abstract
Soil organic carbon (SOC) can be defined by measurable chemical and physical pools, such as mineral-associated carbon, carbon physically entrapped in aggregates, dissolved carbon, and fragments of plant detritus. Yet, most soil models use conceptual rather than measurable SOC pools. What would the traditional pool-based soil model look like if it were built today, reflecting the latest understanding of biological, chemical, and physical transformations in soils? We propose a conceptual model—the Millennial model—that defines pools as measurable entities. First, we discuss relevant pool definitions conceptually and in terms of the measurements that can be used to quantify pool size, formation, and destabilization. Then, we develop a numerical model following the Millennial model conceptual framework to evaluate against the Century model, a widely-used standard for estimating SOC stocks across space and through time. The Millennial model predicts qualitatively similar changes in total SOC in response to single factor perturbations when compared to Century, but different responses to multiple factor perturbations. We review important conceptual and behavioral differences between the Millennial and Century modeling approaches, and the field and lab measurements needed to constrain parameter values. We propose the Millennial model as a simple but comprehensive framework to model SOC pools and guide measurements for further model development.






Similar content being viewed by others
References
Abramoff RZ, Finzi AC (2016) Seasonality and partitioning of root allocation to rhizosphere soils in a midlatitude forest. Ecosphere. https://doi.org/10.1002/ecs2.1547
Abramoff RZ, Davidson EA, Finzi AC (2017) A parsimonious modular approach to building a mechanistic belowground carbon and nitrogen model. J Geophys Res Biogeosci 122:2418–2434
Ahrens B, Braakhekke MC, Guggenberger G et al (2015) Contribution of sorption, DOC transport and microbial interactions to the 14C age of a soil organic carbon profile: insights from a calibrated process model. Soil Biol Biochem 88:390–402
Albalasmeh AA, Ghezzehei TA (2013) Interplay between soil drying and root exudation in rhizosheath development. Plant Soil 374:739–751
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry 81:361–373
Allison SD, Jastrow JD (2006) Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol Biochem 38:3245–3256
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Averill C (2014) Divergence in plant and microbial allocation strategies explains continental patterns in microbial allocation and biogeochemical fluxes. Ecol Lett. https://doi.org/10.1111/ele.12324
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bailey VL, Bond-Lamberty B, DeAngelis K et al (2017) Soil carbon cycling proxies: understanding their critical role in predicting climate change feedbacks. Glob Change Biol 00:1–11
Baker NR, Allison SD (2015) Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology 96:1994–2003
Blankinship JC, Fonte SJ, Six J, Schimel JP (2016) Plant versus microbial controls on soil aggregate stability in a seasonally dry ecosystem. Geoderma 272:39–50
Boddy E, Hill P, Farrar J, Jones D (2007) Fast turnover of low molecular weight components of the dissolved organic carbon pool of temperate grassland field soils. Soil Biol Biochem 39:827–835
Bonan GB, Hartman MD, Parton WJ, Wieder WR (2013) Evaluating litter decomposition in earth system models with long-term litterbag experiments: an example using the Community Land Model version 4 (CLM4). Glob Change Biol 19:957–974
Bradford MA, Wieder WR, Bonan GB et al (2016) Managing uncertainty in soil carbon feedbacks to climate change. Nat Clim Change 6:751–758
Burns RG, DeForest JL, Marxsen J et al (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Cai A, Feng W, Zhang W, Xu M (2016) Climate, soil texture, and soil types affect the contributions of fine-fraction-stabilized carbon to total soil organic carbon in different land uses across China. J Environ Manag 172:2–9
Castro HF, Classen AT, Austin EE et al (2010) Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microbiol 76:999–1007
Chenu C, Plante AF (2006) Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the “primary organo-mineral complex”. Eur J Soil Sci 57:596–607
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995
Cotrufo MF, Soong JL, Horton AJ et al (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779
Crawford JW, Deacon L, Grinev D et al (2012) Microbial diversity affects self-organization of the soil–microbe system with consequences for function. J R Soc Interface 9:1302–1310
Davidson EA, Samanta S, Caramori SS, Savage K (2012) The Dual Arrhenius and Michaelis–Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob Change Biol 18:371–384
De Gryze S, Six J, Merckx R (2006) Quantifying water–stable soil aggregate turnover and its implication for soil organic matter dynamics in a model study. Eur J Soil Sci 57:693–707
DeAngelis KM, Pold G, Topçuoğlu BD et al (2015) Long-term forest soil warming alters microbial communities in temperate forest soils. Front Microbiol 6:104
Del Grosso SJ, Parton WJ, Mosier AR et al (2005) Modeling soil CO2 emissions from ecosystems. Biogeochemistry 73:71–91
Denef K, Six J, Merckx R, Paustian K (2002) Short-term effects of biological and physical forces on aggregate formation in soils with different clay mineralogy. Plant Soil 246:185–200
Devêvre OC, Horwáth WR (2000) Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol Biochem 32:1773–1785
Dexter AR (1988) Advances in characterization of soil structure. Soil Tillage Res 11:199–238
Dwivedi D, Riley WJ, Torn MS, et al (2017) Mineral properties, microbes, transport, and plant-input profiles control vertical distribution and age of soil carbon stocks. Soil Biol Biochem 107:244–259
Ekschmitt K, Liu M, Vetter S et al (2005) Strategies used by soil biota to overcome soil organic matter stability—why is dead organic matter left over in the soil? Geoderma 128:167–176
Fahey TJ, Siccama TG, Driscoll CT et al (2005) The biogeochemistry of carbon at Hubbard Brook. Biogeochemistry 75:109–176
Feng W, Klaminder J, Boily J-F (2015) Thermal stability of goethite-bound natural organic matter is impacted by carbon loading. J Phys Chem A 119:12790–12796
Feng W, Shi Z, Jiang J et al (2016) Methodological uncertainty in estimating carbon turnover times of soil fractions. Soil Biol Biochem 100:118–124
Fontaine S, Barot S, Barré P et al (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Frey SD, Lee J, Melillo JM, Six J (2013) The temperature response of soil microbial efficiency and its feedback to climate. Nat Clim Change 3:395–398
Georgiou K, Abramoff RZ, Harte J et al (2017) Microbial community-level regulation explains soil carbon responses to long-term litter manipulations. Nat Commun 8:1223
Gerke HH (2006) Preferential flow descriptions for structured soils. Z Pflanzenernähr Bodenkd 169:382–400
German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis–Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob Change Biol 18:1468–1479
Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD (2016) Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 127:173–188
Grant RF (2001) A review of Canadian ecosystem model—ecosys. In: Modeling carbon and nitrogen dynamics for soil management, p 173–264. https://doi.org/10.1201/9781420032635.ch6
Grant RF (2013) Modelling changes in nitrogen cycling to sustain increases in forest productivity under elevated atmospheric CO2 and contrasting site conditions. Biogeosciences 10:7703–7721
Hall SJ, McNicol G, Natake T, Silver WL (2015) Large fluxes and rapid turnover of mineral-associated carbon across topographic gradients in a humid tropical forest: insights from paired 14C analysis. Biogeosciences 12:2471–2487
Hanson PJ, Gill AL, Xu X et al (2016) Intermediate-scale community-level flux of CO2 and CH4 in a Minnesota peatland: putting the SPRUCE project in a global context. Biogeochemistry 129:255–272
Hararuk O, Obrist D, Luo Y (2013) Modelling the sensitivity of soil mercury storage to climate-induced changes in soil carbon pools. Biogeosciences 10:2393–2407
Heckman K, Throckmorton H, Clingensmith C et al (2014) Factors affecting the molecular structure and mean residence time of occluded organics in a lithosequence of soils under ponderosa pine. Soil Biol Biochem 77:1–11
Horn R, Taubner H, Wuttke M, Baumgartl T (1994) Soil physical properties related to soil structure. Soil Tillage Res 30:187–216
Jagadamma S, Mayes MA, Phillips JR (2012) Selective sorption of dissolved organic carbon compounds by temperate soils. PLoS ONE. https://doi.org/10.1371/journal.pone.0050434
Jagadamma S, Megan Steinweg J, Mayes MA et al (2013) Decomposition of added and native organic carbon from physically separated fractions of diverse soils. Biol Fertil Soils 50:613–621
Jardine PM, McCarthy JF (1989) Mechanisms of dissolved organic carbon adsorption on soil. https://doi.org/10.2136/sssaj1989.03615995005300050013x
Jardine PM, Mayes MA, Mulholland PJ et al (2006) Vadose zone flow and transport of dissolved organic carbon at multiple scales in humid regimes. Vadose Zone J 5:140–152
Jastrow JD, Miller RM, Boutton TW (1996) Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance. Soil Sci Soc Am J 60:801
Jastrow JD, Miller RM, Lussenhop J (1998) Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol Biochem 30:905–916
Jenkinson DS, Coleman K (2008) The turnover of organic carbon in subsoils. Part 2. Modelling carbon turnover. Eur J Soil Sci 59:400–413
Junicke H, Abbas B, Oentoro J et al (2014) Absolute quantification of individual biomass concentrations in a methanogenic coculture. AMB Express. https://doi.org/10.1186/s13568-014-0035-x
Kaiser K, Kalbitz K (2012) Cycling downwards—dissolved organic matter in soils. Soil Biol Biochem 52:29–32
Kaiser K, Guggenberger G, Zech W (1996) Sorption of DOM and DOM fractions to forest soils. Geoderma 74:281–303
Kalbitz K, Kaiser K (2008) Contribution of dissolved organic matter to carbon storage in forest mineral soils. Z Pflanzenernähr Bodenkd 171:52–60
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630
Kleber M, Sollins P, Sutton R (2007) A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85:9–24
Kleber M, Nico PS, Plante A et al (2011) Old and stable soil organic matter is not necessarily chemically recalcitrant: implications for modeling concepts and temperature sensitivity. Glob Change Biol 17:1097–1107
Kothawala DN, Moore TR, Hendershot WH (2009) Soil properties controlling the adsorption of dissolved organic carbon to mineral soils. Soil Sci Soc Am J 73:1831–1842
Koven CD, Riley WJ, Subin ZM et al (2013) The effect of vertically resolved soil biogeochemistry and alternate soil C and N models on C dynamics of CLM4. Biogeosciences 10:7109–7131
Lajtha K, Bowden RD, Nadelhoffer K (2014a) Twenty years of litter and root manipulations in a temperate deciduous forest: Insights into soil organic matter dynamics and stability. Soil Sci Soc Am J 78:261–269
Lajtha K, Townsend KL, Kramer MG et al (2014b) Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 119:341–360
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68
Liao JD, Boutton TW, Jastrow JD (2006) Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol Biochem 38:3184–3196
Luo Y, Ahlström A, Allison SD et al (2015) Towards more realistic projections of soil carbon dynamics by earth system models. Glob Biogeochem Cycles. https://doi.org/10.1002/2015gb005239
Manzoni S, Porporato A (2009) Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol Biochem 41:1355–1379
Manzoni S, Schaeffer SM, Katul G et al (2014) A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol Biochem 73:69–83
Marin-Spiotta E, Silver WL, Swanston CW, Ostertag R (2009) Soil organic matter dynamics during 80 years of reforestation of tropical pastures. Glob Change Biol 15:1584–1597
Martin JP, Martin WP, Page JB et al (1955) Soil aggregation. Adv Agron 7:1–37
Mayer LM (1994) Relationships between mineral surfaces and organic carbon concentrations in soils and sediments. Chem Geol 114:347–363
Mayes MA, Heal KR, Brandt CC et al (2012) Relation between soil order and sorption of dissolved organic carbon in temperate subsoils. Soil Sci Soc Am J 76:1027–1037
McCarthy JF, Ilavsky J, Jastrow JD et al (2008) Protection of organic carbon in soil microaggregates via restructuring of aggregate porosity and filling of pores with accumulating organic matter. Geochim Cosmochim Acta 72:4725–4744
Melillo JM, Butler S, Johnson J et al (2011) Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 108:9508–9512
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141
Norby RJ, Luo Y (2004) Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. N Phytol 162:281–293
O’Brien SL, Jastrow JD (2013) Physical and chemical protection in hierarchical soil aggregates regulates soil carbon and nitrogen recovery in restored perennial grasslands. Soil Biol Biochem 61:1–13
O’Brien SL, Jastrow JD, McFarlane KJ et al (2013) Decadal cycling within long-lived carbon pools revealed by dual isotopic analysis of mineral-associated soil organic matter. Biogeochemistry 112:111–125
Oleson KW, Lawrence DM, Bonan GB et al (2013) Technical description of version 4.5 of the Community Land Model (CLM). NCAR Tech. National Center for Atmospheric Research, Bounder
Parton WJ, Schimel DS, Cole CV et al (1987) Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci Soc Am J 51:1173–1179
Parton WJ, Scurlock JMO, Ojima DS et al (1995) Impact of climate change on grassland production and soil carbon worldwide. Glob Change Biol 1:13–22
Parton WJ, Hartman M, Ojima D, Schimel D (1998) DAYCENT and its land surface submodel: description and testing. Glob Planet Change 19:35–48
Parton WJ, Hanson PJ, Swanston C et al (2010) ForCent model development and testing using the Enriched Background Isotope Study experiment. J Geophys Res. https://doi.org/10.1029/2009jg001193
Paustian K, Parton WJ, Persson J (1992) Modeling soil organic matter in organic-amended and nitrogen-fertilized long-term plots. Soil Sci Soc Am J 56:476–488
Plante AF, Conant RT, Paul EA et al (2006) Acid hydrolysis of easily dispersed and microaggregate-derived silt- and clay-sized fractions to isolate resistant soil organic matter. Eur J Soil Sci 57:456–467
Pronk GJ, Heister K, Ding G-C et al (2012) Development of biogeochemical interfaces in an artificial soil incubation experiment; aggregation and formation of organo-mineral associations. Geoderma 189–190:585–594
Ranjard L, Richaume A (2001) Quantitative and qualitative microscale distribution of bacteria in soil. Res Microbiol 152:707–716
Riley WJ, Maggi F, Kleber M et al (2014) Long residence times of rapidly decomposable soil organic matter: application of a multi-phase, multi-component, and vertically resolved model (BAMS1) to soil carbon dynamics. Geosci Model Dev 7:1335–1355
Rumpel C, Eusterhues K, Kögel-Knabner I (2010) Non-cellulosic neutral sugar contribution to mineral associated organic matter in top- and subsoil horizons of two acid forest soils. Soil Biol Biochem 42:379–382
Schimel DS (1995) Terrestrial ecosystems and the carbon cycle. Glob Change Biol. https://doi.org/10.1111/j.1365-2486.1995.tb00008.x
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Segoli M, De Gryze S, Dou F et al (2013) AggModel: a soil organic matter model with measurable pools for use in incubation studies. Ecol Model 263:1–9
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Sierra CA, Trumbore SE, Davidson EA et al (2012) Predicting decadal trends and transient responses of radiocarbon storage and fluxes in a temperate forest soil. https://doi.org/10.5194/bg-9-3013-2012
Sierra CA, Trumbore SE, Davidson EA et al (2015) Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J Adv Model Earth Syst 7:335–356
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Belnap J, Findlay SG et al (2014a) Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 121:287–304
Sinsabaugh RL, Follstad Shah JJ, Findlay SG et al (2014b) Scaling microbial biomass, metabolism and resource supply. Biogeochemistry 122:175–190
Sinsabaugh RL, Moorhead DL, Xu X, Litvak ME (2017) Plant, microbial and ecosystem carbon use efficiencies interact to stabilize microbial growth as a fraction of gross primary production. N Phytol. https://doi.org/10.1111/nph.14485
Sistla SA, Rastetter EB, Schimel JP (2014) Responses of a tundra system to warming using SCAMPS: a stoichiometrically coupled, acclimating microbe–plant–soil model. Ecol Monogr 84:151–170
Six J, Paustian K (2014) Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol Biochem 68:A4–A9
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099–2103
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555
Smith AP, Bond-Lamberty B, Benscoter BW et al (2017) Shifts in pore connectivity from precipitation versus groundwater rewetting increases soil carbon loss after drought. Nat Commun. https://doi.org/10.1038/s41467-017-01320-x
Sollins P, Homann P, Caldwell BA (1996) Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74:65–105
Sollins P, Kramer MG, Swanston C et al (2009) Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96:209–231
Steinweg JM, Plante AF, Conant RT et al (2008) Patterns of substrate utilization during long-term incubations at different temperatures. Soil Biol Biochem 40:2722–2728
Sulman BN, Phillips RP, Oishi AC et al (2014) Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO 2. Nat Clim Change 4:1099–1102
Suseela V, Conant RT, Wallenstein MD, Dukes JS (2012) Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob Change Biol 18:336–348
Tang JY (2015) On the relationships between Michaelis–Menten kinetics, reverse Michaelis–Menten kinetics, Equilibrium Chemistry Approximation kinetics and quadratic kinetics. Geosci Model Dev Discuss 8:7663–7691
Tang J, Riley WJ (2015) Weaker soil carbon–climate feedbacks resulting from microbial and abiotic interactions. Nat Clim Change. https://doi.org/10.1038/nclimate2438
Thornton PE, Lamarque J-F, Rosenbloom NA, Mahowald NM (2007) Influence of carbon–nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob Biogeochem Cycles. https://doi.org/10.1029/2006gb002868
Tisdall J, Oades J (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Todd-Brown KEO, Hopkins FM, Kivlin SN et al (2011) A framework for representing microbial decomposition in coupled climate models. Biogeochemistry 109:19–33
Todd-Brown KEO, Randerson JT, Post WM, et al (2013) Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations
Todd-Brown KEO, Randerson JT, Hopkins F et al (2014) Changes in soil organic carbon storage predicted by Earth system models during the 21st century. Biogeosciences 11:2341–2356
Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM (1997) Mineral control of soil organic carbon storage and turnover. Nature 389:170–173
Torn MS, Swanston CW, Castanha C, Trumbore SE (2009) Storage and turnover of organic matter in soil. In: Biophysico-chemical processes involving natural nonliving organic matter in environmental systems. Wiley, Hoboken, p 219–272
van Ginkel JH, Gorissen A, Polci D (2000) Elevated atmospheric carbon dioxide concentration: effects of increased carbon input in a Lolium perenne soil on microorganisms and decomposition. Soil Biol Biochem 32:449–456
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Virto I, Barré P, Chenu C (2008) Microaggregation and organic matter storage at the silt-size scale. Geoderma 146:326–335
von Lützow M, Kögel-Knabner I, Ekschmitt K et al (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207
Wang G, Post WM, Mayes MA (2013) Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecol Appl 23:255–272
Wang YP, Jiang J, Chen-Charpentier B et al (2016) Responses of two nonlinear microbial models to warming and increased carbon input. Biogeosciences 13:887–902
Wershaw RL (1986) A new model for humic materials and their interactions with hydrophobic organic chemicals in soil–water or sediment–water systems. J Contam Hydrol 1:29–45
Wieder WR, Bonan GB, Allison SD (2013) Global soil carbon projections are improved by modelling microbial processes. Nat Clim Change 3:1–7
Wieder WR, Grandy AS, Kallenbach CM, Bonan GB (2014) Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 11:3899–3917
Wieder WR, Allison SD, Davidson EA et al (2015a) Explicitly representing soil microbial processes in Earth system models. Glob Biogeochem Cycles 29:1782–1800
Wieder WR, Grandy AS, Kallenbach CM et al (2015b) Representing life in the Earth system with soil microbial functional traits in the MIMICS model. Geosci Model Dev Discuss 8:2011–2052
Xu X, Schimel JP, Thornton PE et al (2014) Substrate and environmental controls on microbial assimilation of soil organic carbon: a framework for Earth system models. Ecol Lett 17:547–555
Young IM, Crawford JW (2004) Interactions and self-organization in the soil–microbe complex. Science 304:1634–1637
Young IM, Crawford JW, Nunan N, et al (2008) Chapter 4 Microbial Distribution in Soils: Physics and Scaling. In: Advances in Agronomy. Academic Press, pp 81–121
Zaehle S, Medlyn BE, De Kauwe MG et al (2014) Evaluation of 11 terrestrial carbon–nitrogen cycle models against observations from two temperate Free-Air CO2 Enrichment studies. N Phytol 202:803–822
Zhuang J, McCarthy JF, Perfect E et al (2008) Soil water hysteresis in water-stable microaggregates as affected by organic matter. Soil Sci Soc Am J 72:212–220
Acknowledgements
The Millennial model code, model inputs, and the model output used in this manuscript are archived at a GITHUB Repository (https://github.com/email-clm/Millennial) that is publicly accessible. The authors would like to thank the Carbon Cycle Interagency Working Group, via the US Carbon Cycle Science Program under the auspices of the US Global Change Research Program, for providing funding for the “Celebrating the 2015 International Decade of Soil – Understanding Soil’s Resilience and Vulnerability,” workshop held at the University Corporation for Atmospheric Research in Boulder, CO, USA on 14–16 March 2016. We would also like to thank the University Corporation for Atmospheric Research for providing meeting space, as well as the 36 workshop participants, William J. Riley, and three anonymous reviewers for helpful comments and discussion. Lawrence Berkeley National Laboratory is managed and operated by the Regents of the University of California under Contract DE-AC02-05CH11231 with the US Department of Energy. Argonne National Laboratory is managed by UChicago Argonne, LLC, under contract DE-AC02-06CH11357 with the US Department of Energy. Oak Ridge National Laboratory is managed by the University of Tennessee-Battelle, LLC, under Contract DE-AC05-00OR22725 with the US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Marc G. Kramer.
The submitted manuscript has been authored by contractors of the US Government under contracts DE-AC02-05CH11231 (LBNL), DE-AC02-06CH11357 (ANL), and DE-AC05-00OR22725 (ORNL). Accordingly, the US Government retains a nonexclusive, royalty-free license to publish or reproduce the published form of this contribution, or allow others to do so, for US Government purposes.
Appendix: Model description
Appendix: Model description
The equations we have chosen below reflect one possible mathematical expression of the Millennial conceptual model, but there are many possible numerical models for different applications. For example, decomposition of POM is here represented by a double Monod relationship, limited by both POM and microbial biomass, but for an application where competition between chemical species is particularly important, for example, ECA kinetics could be used instead (Tang 2015). Similarly, we chose temperature and moisture scalars to minimize steady-state differences between Millennial and Century for the purpose of model comparison, but for dynamic predictions one could apply Arrhenius temperature sensitivity and one of several semi-mechanistic moisture functions (Davidson et al. 2012; Manzoni et al. 2014).
The system of equations below is modeled on the conceptual figure (Fig. 1), tracking the size of and transfers between five C pools: POM, LMWC, aggregate C, MAOM, and microbial biomass. The change in POM (P) stock with time is governed by the balance between plant C input and aggregate C breakdown, aggregate C formation, and decomposition,
where F i is aboveground plant litter, root litter and root exudates, p i is the proportion of C input allocated to POM (1/3 of inputs to POM and 2/3 of inputs to LMWC after Oleson et al. 2013), p a is the proportion of C in aggregate breakdown allocated to POM, F a is aggregate C breakdown, F pa is aggregate carbon formation from POM, and F pl is decomposition of POM into LMWC. Decomposition of POM is governed by a double Michaelis–Menten equation,
where V pl is the maximum rate of POM decomposition, K pl is the half-saturation constant, B is the microbial biomass carbon, and K pe is the half-saturation constant of microbial control on POM mineralization. The terms S t and S w refer to the temperature and moisture scalar, respectively, and are taken from DAYCENT, the daily time-step version of the Century model (Parton et al. 1998), to minimize differences in temperature and moisture effects between the Century and Millennial models due to choice of scalar,
where T is the current temperature, T ref is the reference temperature, t 1 is the x-axis location of the inflection point (°C), t 2 is the y-axis location of the inflection point, t 3 is the distance from the maximum point to the minimum point, and t 4 is the slope of the line at the inflection point. For the water scalar, RWC is the relative water content calculated as the fraction of field capacity, and w 1 and w 2 are empirical parameters. The temperature scalar is an arctangent function that predicts a decline in temperature sensitivity with increasing temperature and the water scalar depends on RWC, where the maximum effect on biological activity occurs at field capacity (volumetric water content = 0.35, RWC = 1.0) (Parton et al. 2010).
The formation of aggregate C (A) from POM follows Michaelis–Menten dynamics,
where V pa is the maximum rate of aggregate formation, K pa is the half-saturation constant of aggregate formation, and A max is the maximum capacity of C in soil aggregates. Soil aggregate C breakdown is partitioned to POM and MAOM,
where k b is the rate of breakdown.
The change in LMWC (L) depends on LMWC input, the leaching rate, decomposition of POM, adsorption to minerals, and microbial uptake. In a multilayer version of the Millennial model LMWC would also depend on leaching input, but in this single layer version we assume that the leaching input is included in the LMWC input,
where F l is the LMWC leaching loss,
and where k l is the leaching rate, F lm is the adsorption of LMWC to MAOM, and F lb is the uptake of LMWC by microbial biomass. Adsorption of LMWC to minerals is controlled by a Langmuir saturation function,
where K lm is the binding affinity that is adjustable based on the pH. Q max is the maximum sorption capacity (mg C kg−1 dry soil) that is converted to C density (g C m−2) by multiplying soil bulk density (BD = 1350 kg m−3), assuming a 1 m soil profile. The parameters c 1 and c 2 are the coefficients for computing Q max from the clay content in percent, derived from Mayes et al. (2012). The Langmuir function parameters were derived from measurements of DOC sorption on over 200 soils in the eastern US. The measurements demonstrate a nonlinear saturation with respect to DOC concentrations in soils, and several recent models have used approaches that also impose a mechanism for DOC saturation on mineral surfaces (Wang et al. 2013; Riley et al. 2014; Ahrens et al. 2015; Dwivedi et al. 2017).
Microbial uptake of LMWC is a function of microbial biomass and LMWC concentration, temperature, water, and temperature-dependent CUE,
where Vlm is the potential uptake rate of LMWC. F gr is microbial growth-related respiration, Klb is the half-saturation constant for microbial activity, CUEref is the reference CUE, and CUET is the CUE dependence on temperature. Tae-ref and T are the reference and current temperature, respectively. Both MAOM and POM can be incorporated into the aggregate C pool,
where F ma is the carbon flow from MAOM to aggregate C, V ma is the maximum rate of aggregate formation, and K ma is the half-saturation constant of aggregate formation. MAOM is formed by adsorption of LMWC and microbial necromass, and is affected by transfer into and out of the aggregate C pool,
where F bm is the carbon flow from microbial biomass to MAOM, namely adsorption of necromass, and k mm is the adsorption rate of microbial biomass. In this particular iteration of the Millennial model, we assume that adsorbed microbial biomass is no longer alive, but by allowing adsorbed microbial biomass to take up LMWC and perform growth and maintenance, one could modify the model to accommodate the assumption that live microbial biomass can sorb to minerals, or even to other microbes (i.e., biofilms). Microbial biomass changes as a result of uptake, adsorption to minerals, and loss via maintenance,
where F mr is the maintenance respiration of microbial biomass, and k m is the microbial turnover rate (Table 3).
Rights and permissions
About this article
Cite this article
Abramoff, R., Xu, X., Hartman, M. et al. The Millennial model: in search of measurable pools and transformations for modeling soil carbon in the new century. Biogeochemistry 137, 51–71 (2018). https://doi.org/10.1007/s10533-017-0409-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-017-0409-7