Abstract
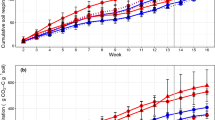
Climate models project that precipitation patterns will likely intensify in the future, resulting in increased duration of droughts and increased frequency of large soil rewetting events, which are stressful to the microorganisms that drive soil biogeochemical cycling. Historical conditions can affect contemporary microbial responses to environmental factors through the persistence of abiotic changes or through the selection of a more tolerant microbial community. We examined how a history of intensified rainfall would alter microbial functional response to drying and rewetting events, whether this historical legacy was mediated through altered microbial community composition, and how long community and functional legacies persisted under similar conditions. We collected soils from a long-term field manipulation (Rainfall Manipulation Plot Study) in Kansas, USA, where rainfall variability was experimentally amplified. We measured respiration, microbial biomass, fungal:bacterial ratios and bacterial community composition after collecting soils from the field experiment, and after subjecting them to a series of drying–rewetting pulses in the lab. Although rainfall history affected respiration and microbial biomass, the differences between field treatments did not persist throughout our 115-day drying–rewetting incubation. However, soils accustomed to more extreme rainfall did change less in response to lab moisture pulses. In contrast, bacterial community composition did not differ between rainfall manipulation treatments, but became more dissimilar in response to drying–rewetting pulses depending on their previous field conditions. Our results suggest that environmental history can affect contemporary rates of biogeochemical processes both through changes in abiotic drivers and through changes in microbial community structure. However, the extremity of the disturbance and the mechanism through which historical legacies occur may influence how long they persist, which determines the importance of these effects for biogeochemical cycling.







Similar content being viewed by others
References
Adu JK, Oades JM (1978) Physical factors influencing decomposition of organic materials in soil aggregates. Soil Biol Biochem 10(2):109–115. doi:10.1016/0038-0717(78)90080-9
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA 105:11512–11519. doi:10.1073/pnas.0801925105
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26(1):32–46. doi:10.1046/j.1442-9993.2001.01070.x
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141(2):221–235. doi:10.1007/s00442-004-1519-1
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41(3):606–610. doi:10.1016/j.soilbio.2008.12.022
Bapiri A, Baath E, Rousk J (2010) Drying–rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb Ecol 60(2):419–428. doi:10.1007/s00248-010-9723-5
Birch H (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 12:9–31
Brown JR, Blankinship JC, Niboyet A, van Groenigen CJ, Dijkstra P, Leadley PW, Hungate BA (2011) Effects of multiple global change treatments on soil N2O fluxes. Biogeochemistry. doi:10.1007/s10533-011-9655-2
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010a) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26(2):266–267. doi:10.1093/bioinformatics/btp636
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010b) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. doi:10.1038/nmeth.f.303
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Collins SL, Sinsabaugh RL, Crenshaw C, Green L, Porras-Alfaro A, Stursova M, Zeglin LH (2008) Pulse dynamics and microbial processes in aridland ecosystems. J Ecol 96(3):413–420. doi:10.1111/j.1365-2745.2008.01362.x
Cruz-Martinez K, Suttle KB, Brodie EL, Power ME, Andersen GL, Banfield JF (2009) Despite strong seasonal responses, soil microbial consortia are more resilient to long-term changes in rainfall than overlying grassland. ISME J 3(6):738–744. http://www.nature.com/ismej/journal/v3/n6/suppinfo/ismej200916s1.html
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic-stress. Microbiol Rev 53(1):121–147
De Angelis KM, Silver WL, Thompson AW, Firestone MK (2010) Microbial communities acclimate to recurring changes in soil redox potential status. Environ Microbiol 12(12):3137–3149. doi:10.1111/j.1462-2920.2010.02286.x
de Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29(4):795–811. doi:10.1016/j.femsre.2004.11.005
Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2000) Altering rainfall timing and quantity in a mesic grassland ecosystem: design and performance of rainfall manipulation shelters. Ecosystems 3(3):308–319
Fay PA, Carlisle JD, Danner BT, Lett MS, McCarron JK, Stewart C, Knapp AK, Blair JM, Collins SL (2002) Altered rainfall patterns, gas exchange, and growth in grasses and forbs. Int J Plant Sci 163(4):549–557
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34(6):777–787
Fierer N, Schimel JP, Holden PA (2003) Influence of drying–rewetting frequency on soil bacterial community structure. Microb Ecol 45(1):63–71. doi:10.1007/s00248-002-1007-2
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88(6):1354–1364
Fierer N, Hamady M, Lauber CL, Knight R (2008) The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 105(46):17994–17999. doi:10.1073/pnas.0807920105
Gardes M, Bruns TD (1993) Its primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118
Gonzalez-Quinones V, Banning NC, Ballesta RJ, Murphy DV (2009) Influence of cold storage on soil microbial community level physiological profiles and implications for soil quality monitoring. Soil Biol Biochem 41(7):1574–1576. doi:10.1016/j.soilbio.2009.04.004
Gordon H, Haygarth PM, Bardgett RD (2008) Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol Biochem 40(2):302–311
Gulledge J, Schimel JP (1998) Moisture control over atmospheric CH4 consumption and CO2 production in diverse Alaskan soils. Soil Biol Biochem 30(8–9):1127–1132
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R (2008) Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5(3):235–237. doi:10.1038/nmeth.1184
Harper CW, Blair JM, Fay PA, Knapp AK, Carlisle JD (2005) Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob Change Biol 11(2):322–334. doi:10.1111/j.1365-2486.2005.00899.x
Harris RF (1981) Effect of water potential on microbial growth and activity. In: Parr JF, Gardner WR, Elliott LF (eds) Water potential relations in soil microbiology. American Society of Agronomy, Madison, pp 23–95
Hawkes CV, Kivlin SN, Rocca JD, Huguet V, Thomsen MA, Suttle KB (2010) Fungal community responses to precipitation. Glob Change Biol 17:1637–1645. doi:10.1111/j.1365-2486.2010.02327.x
Huntington TG (2006) Evidence for intensification of the global water cycle: review and synthesis. J Hydrol 319(1–4):83–95. doi:10.1016/j.jhydrol.2005.07.003
Jenny H (1941) Factors of soil formation: a system of quantitative pedology. McGraw-Hill, New York
Keiser AD, Strickland MS, Fierer N, Bradford MA (2010) The effect of resource history on the functioning of soil microbial communities is maintained across time. Biogeosciences 8(6):1477–1486. doi:10.5194/bg-8-1477-2011
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron JK (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298(5601):2202–2205
Landesman WJ, Dighton J (2010) Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol Biochem 42(10):1751–1758. doi:10.1016/j.soilbio.2010.06.012
Lane D (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematic. John Wiley, New York, pp 115–117
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75(15):5111–5120. doi:10.1128/aem.00335-09
Lawrence CR, Neff JC, Schimel JP (2009) Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol Biochem 41(9):1923–1934. doi:10.1016/j.soilbio.2009.06.016
Lee YB, Lorenz N, Dick LK, Dick RP (2007) Cold storage and pretreatment incubation effects on soil microbial properties. Soil Sci Soc Am J 71(4):1299–1305. doi:10.2136/sssaj2006.0245
Li XY, Miller AE, Meixner T, Schimel JP, Melack JM, Sickman JO (2010) Adding an empirical factor to better represent the rewetting pulse mechanism in a soil biogeochemical model. Geoderma 159(3–4):440–451. doi:10.1016/j.geoderma.2010.09.012
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235. doi:10.1128/aem.71.12.8228-8235.2005
Lundquist EJ, Scow KM, Jackson LE, Uesugi SL, Johnson CR (1999) Rapid response of soil microbial communities from conventional, low input, and organic farming systems to a wet/dry cycle. Soil Biol Biochem 31(12):1661–1675
McGill BM, Sutton-Grier AE, Wright JP (2010) Plant trait diversity buffers variability in denitrification potential over changes in season and soil conditions. Plos One 5(7):e11618. doi:10.1371/journal.pone.0011618
McGuire KL, Bent E, Borneman J, Majumder A, Allison SD, Treseder KK (2010) Functional diversity in resource use by fungi. Ecology 91(8):2324–2332. doi:10.1890/09-0654.1
McMahon SK, Wallenstein MD, Schimel JP (2011) A cross-seasonal comparison of active and total bacterial community composition in Arctic tundra soil using bromodeoxyuridine labeling. Soil Biol Biochem 43(2):287–295. doi:10.1016/j.soilbio.2010.10.013
Miller AE, Schimel JP, Meixner T, Sickman JO, Melack JM (2005) Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol Biochem 37(12):2195–2204. doi:10.1016/j.soilbio.2005.03.021
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial-populations by denaturing gradient gel-electrophoresis analysis of polymerase chain reaction-amplified genes-coding for 16s ribosomal-RNA. Appl Environ Microbiol 59(3):695–700
Schimel J (1995) Ecosystem consequences of microbial diversity and community structure. In: Chapin FSI, Koerner C (eds) Arctic and alpine biodiversity: patterns, causes and ecosystem consequences. Springer, New York, pp 239–254
Schimel JP, Gulledge JM, Clein-Curley JS, Lindstrom JE, Braddock JF (1999) Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol Biochem 31(6):831–838
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88(6):1386–1394
Stres B, Philippot L, Faganeli J, Tiedje JM (2010) Frequent freeze–thaw cycles yield diminished yet resistant and responsive microbial communities in two temperate soils: a laboratory experiment. FEMS Microbiol Ecol 74(2):323–335. doi:10.1111/j.1574-6941.2010.00951.x
Tobor-Kaplon MA, Bloem J, De Ruiter PC (2006) Functional stability of microbial communities from long-term stressed soils to additional disturbance. Environ Toxicol Chem 25(8):1993–1999
Todd-Brown KEO, Hopkins FM, Kivlin SN, Talbot JM, Allison SD (2011) A framework for representing microbial decomposition in coupled climate models. Biogeochemistry. doi:10.1007/s10533-011-9635-6
Treseder KK, Balser TC, Bradford MA, Brodie EL, Dubinsky EA, Eviner VT, Hofmockel KS, Lennon JT, Levine UY, MacGregor BJ, Pett-Ridge J, Waldrop MP (2011) Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry. doi:10.1007/s10533-011-9636-5
Van Gestel M, Merckx R, Vlassak K (1993) Microbial biomass responses to soil drying and rewetting: the fate of fast- and slow-growing microorganisms in soils from different climates. Soil Biol Biochem 25(1):109–123
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19(6):703–707
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several cryptococcus species. J Bacteriol 172(8):4238–4246
Waldrop MP, Firestone MK (2006) Response of microbial community composition and function to soil climate change. Microb Ecol 52(4):716–724. doi:10.1007/s00248-006-9103-3
Wallenstein MD, Hall EK (2011) A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry. doi:10.1007/s10533-011-9641-8
Wallenstein MD, Myrold DD, Firestone M, Voytek M (2006) Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods. Ecol Appl 16(6):2143–2152. doi:10.1890/1051-0761(2006)016[2143:ecodca]2.0.co;2
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. doi:10.1128/aem.00062-07
Wood JM, Bremer E, Csonka LN, Kraemer R, Poolman B, van der Heide T, Smith LT (2001) Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A 130(3):437–460
Yuste JC, Penuelas J, Estiarte M, Garcia-Mas J, Mattana S, Ogaya R, Pujol M, Sardans J (2010) Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob Change Biol 17(3):1475–1486. doi:10.1111/j.1365-2486.2010.02300.x
Acknowledgments
This research was supported by the U.S. Department of Energy’s Office of Science (BER) through the Midwestern Regional Center of the National Institute for Climatic Change Research to Alan Knapp, John Blair, and Matthew Wallenstein. The RaMPS experiment was implemented with funding from NSF through the Konza LTER, and maintained with support from the NSF LTREB program and USDA Managed Ecosystems. We would like to thank all those involved in executing the RaMPS project at Konza Biological Station, especially Ari Jumpponen and John Blair for assistance collecting soils. We would like to thank Guy Beresford for his assistance with the pyrosequencing, Greg Caparoso for training and advice on QIIME analyses, and Indy Burke, Colin Bell, John Moore, Claudia Boot and two anonymous reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Evans, S.E., Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter?. Biogeochemistry 109, 101–116 (2012). https://doi.org/10.1007/s10533-011-9638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-011-9638-3