Abstract
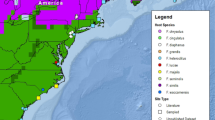
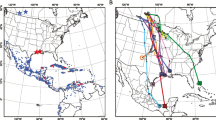
The range of the barred owl (Strix varia) has expanded westward over the past century and now entirely overlaps the range of the federally threatened northern spotted owl (S. occidentalis caurina) in the Pacific Northwest. We compared Haemoproteus blood parasite assemblages among northern spotted owls in their native range and barred owls in both their native and invasive ranges to evaluate predictions of five hypotheses about parasites and biological invasions: (1) Enemy Release, where hosts benefit from a loss of parasites in their invasive range, (2) Enemy of My Enemy, where invasive hosts introduce parasites to naïve native hosts, (3) Parasite Spillback, where invasive hosts act as a new reservoir to native parasites, (4) Increased Susceptibility, where native hosts introduce parasites to naïve invasive hosts, and (5) Dilution Effect, where invasive species act as poor hosts to native parasites and decrease the density of potential hosts in their invasive range. We used haplotype network analyses to identify one haplotype common to both owl species throughout North America, three more haplotypes that appeared to be isolated to the barred owl’s historic range, and a fifth haplotype that was only found in California. Based on infection status and parasite diversity in eastern and western barred owl populations, we found strong support for the Enemy Release Hypothesis. Northern spotted owls had higher parasite diversity and probability of infection than sympatric barred owls, offering some support for the Parasite Spillback and Dilution Effect Hypotheses. Overall, this study demonstrates the complexity of host-parasite relationships and highlights some of the ways in which species’ range expansions may alter such relationships among both invasive and native hosts.


Similar content being viewed by others
References
Atkinson CT, Van Riper CIII (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird-parasite interactions: ecology, evolution, and behavior. Oxford University Press, London, pp 19–48
Bandelt HJ, Forster P, Sykes BC, Richards MB (1995) Mitochondrial portraits of human populations using median networks. Genetics 141:743–753
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2006) GenBank. Nucleic Acids Res 34:D16–D20
Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, McAlpine C (2013) Wildlife disease prevalence in human-modified landscapes. Biol Rev 88:427–442
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cassell DL (2010) BootstrapMania!: re-sampling the SAS® way. In: SAS Institute (ed) Proceedings of the SAS® Global Forum 2010 conference, pp 1–11
Chasar A, Loiseau C, Valkiūnas G, Iezhova T, Smith TB, Sehgal RNM (2009) Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol Ecol 18:4121–4133
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Colwell RK (2005) EstimateS: statistical estimation of species richness and shared species from samples, version 7.5. http://purl.oclc.org/estimates. Accessed 20 March 2013
Crawley MJ (ed) (1997) Plant invasions. Blackwell Science, Oxford
Fiorello C, Deem S, Gompper M, Dubovi E (2004) Seroprevalence of pathogens in domestic carnivores on the border of Madidi National Park, Bolivia. Anim Conserv 7:45–54
Franklin AB, Anderson DR, Gutiérrez RJ, Burnham KP (2000) Climate, habitat quality, and fitness in northern spotted owl populations in northwestern California. Ecol Monogr 70:539–590
Gutiérrez RJ (1989) Hematozoa from the spotted owl. J Wildl Dis 25:614–618
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. J Parasitol 90:797–802
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385
Ishak HD, Dumbacher JP, Anderson NL, Keane JJ, Valkiūnas G, Haig SM, Tell LA, Sehgal RNM (2008) Blood parasites in owls with conservation implications for the spotted owl (Strix occidentalis). PLoS One 3:e2304
Johnsgard PA (1988) North American owls: biology and natural history. Smithsonian Institution Press, Washington
Jovani R, Tella JL (2006) Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol 22:214–218
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology 90:2047–2056
Korpimaki E, Hakkarainen H, Bennet GF (1993) Blood parasites and reproductive success of Tengmalm’s owls: detrimental effects on females but not males? Funct Ecol 7:420–426
Krone O, Priemer J, Streich J, Sommer P, Langgemach T, Lessow O (2001) Haemosporida of birds of prey and owls from Germany. Acta Protozool 40:281–289
Lebarbenchon C, Poulin R, Thomas F (2007) Parasitism, biodiversity, and conservation biology. In: Thomas F, Guegan J-F, Renaud F (eds) Ecology and evolution of parasitism. Oxford University Press, Oxford, pp 149–160
Lewicki KE (2013) Haemosporidian parasites of barred owls (Strix varia) and northern spotted owls (S. occidentalis caurina): investigating the effects of an invasive species on parasite transmission and community dynamics. Thesis, Colorado State University
Librado P, Rozas J (2009) DNAsp v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Livezey KB (2009) Range expansion of barred owls, part I: chronology and distribution. Am Midl Nat 161:49–56
Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol 3:171–177
Magurran A (2004) Measuring biological diversity. Blackwell Science Ltd., Malden
McDonald TL, White GC (2010) A comparison of regression models for small counts. J Wildl Manag 74:514–521
Mellor PS, Boorman J, Baylis M (2000) Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol 45:307–340
Mutlow A, Forbes N (2000) Haemoproteus in raptors: pathogenicity, treatment, and control. In: Proceedings of the Association of Avian Veterinarians, pp 157–163
Nei M, Li J (1989) Variances of the average number of nucleotide substitutions within and between populations. Mol Biol Evol 6:290–300
Paterson RA, Townsend CR, Poulin R, Tompkins DM (2011) Introduced brown trout alternative acanthocephalan infections in native fish. J Anim Ecol 80:990–998
Perkins SL, Schall JJ (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol 88:972–978
Perkins SE, Altizer S, Bjornstad O, Burdon JJ, Clay K, Gomez-Aparicio L, Jeschke JM, Johnson PT, Lafferty KD, Malmstrom CM, Martin P, Power A, Strayer DL, Thrall PH, Uriarte M (2008) Invasion biology and parasitic infections. In: Ostfeld RS, Keesing F, Eviner VT (eds) Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton University Press, Princeton, pp 179–204
Phillips BL, Kelehear C, Pizzatto L, Brown GP, Barton D, Shine R (2010) Parasites and pathogens lag behind their host during periods of host range advance. Ecology 91:872–881
Posada D, Crandall KA (2001) Intraspecific gene genealogies: trees grafting into networks. Trends Ecol Evol 16:37–45
Poulin R, Morand S (2004) Parasite biodiversity. Smithsonian Books, Washington
Poulin R, Paterson RA, Townsend CR, Tomkins DM, Kelly DW (2011) Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw Biol 56:676–688
Power AG, Mitchell CE (2004) Pathogen spillover in disease epidemics. Am Nat 164:S79–S89
Remple JD (2004) Intracellular hematozoa of raptors: a review and update. J Avian Med Surg 18:75–88
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. Proc R Soc Lond B Biol Sci 269:885–892
Ricklefs RE, Swanson BL, Fallon SM, Martinéz-Abraín A, Scheuerlein A, Gray J, Latta SC (2005) Community relationships of avian malaria parasites in southern Missouri. Ecol Monogr 75:543–559
Ritland K (2012) DNADiffer. http://gdc.forestry.ubc.ca/downloads. Accessed 25 July 2013
Sabelis MW, Janssen A, Kant MR (2001) Ecology: the enemy of my enemy is my ally. Science 291:2104–2105
Sanders HL (1968) Marine benthic diversity: a comparative study. Am Nat 102:243–282
SAS Institute Inc (2011) SAS® 9.3 SQL Procedure user’s guide. SAS Institute Inc., Cary, NC
Shannon CE, Weaver W (1962) The mathematical theory of information. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature 163:688
Svensson-Coelho M, Blake JG, Loiselle BA, Penrose AS, Parker PG, Ricklefs RE (2013) Diversity, prevalence, and host specificity of Avian Plasmodium and Haemoproteus in a western amazon assemblage. Ornithol Monogr 76:1–47
Tella JL, Blanco G, Forero MG, Gajón Á, Donázar JA, Hiraldo F (1999) Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc Natl Acad Sci USA 96:1785–1789
Ting TF (1998) The thermal environment of northern spotted owls in northwestern California: possible explanations for use of interior old growth and coastal early successional stage forest. Thesis, Humboldt State University
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
US Fish and Wildlife Service (1990) 50 CFR Part 17 Endangered and threatened wildlife and plants; determination of threatened status for the northern spotted owl; final rule. Fed Regist 55:26114–26194
US Fish and Wildlife Service (2011) Revised recovery plan for the Northern Spotted Owl (Strix occidentalis caurina). US Fish and Wildlife Service, Portland
Valkiūnas G (1996) Ecological implications of hematozoa in birds. Bull Scand Soc Parasitol 6:101–103
van Riper C, van Riper SG, Goff ML, Laird M (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56:327–344
Williamson M (1996) Biological invasions. Chapman & Hall, London
Acknowledgments
We thank Peter Carlson and Jeremy Rockweit of Klamath Biological Research Station; Mark Higley and the Hoopa Valley Tribe; Green Diamond Resource Company; Robert Feamster and Sierra Pacific Industries; and Laurie Clark and the National Council for Air and Stream Improvement, Inc., for allowing us to conduct research on their lands and for assistance in collecting western owl samples. The Avian Conservation Center (South Carolina), Wildcare Foundation (Oklahoma), Avian Haven (Maine), Carolina Raptor Center (North Carolina), Audubon of Florida (Florida), The Raptor Center (Minnesota), Tri-State Bird Rescue & Research (Delaware), and Alabama Raptor Center (Alabama) collected all the eastern barred owl samples analyzed in this study. Additional field and laboratory assistance came from Constanza Toro, Annie Kellner, Matthew Hopken, Nikki Crider, and Jeremy Dertien. Dr. Thomas Gidlewski provided access to his microscope; Nic Berrong assisted in installing and navigating the i-Solution Lite software; and Drs. Ellen Martinsen and Robert Ricklefs provided positive control samples. Finally, Drs. Liba Pejchar, Brian Foy, Ken Burnham, and Ann Hess assisted in study design and analysis and 2 anonymous reviewers provided helpful comments and suggestions that greatly improved our manuscript. This work was conducted under the auspices of the Colorado State University Institutional Animal Care and Use Committee protocol #10-1818A. Funding and additional support for this project was provided by the U.S.D.A. Forest Service Region 5 contract 11-CS-11052007-319 and Colorado State University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lewicki, K.E., Huyvaert, K.P., Piaggio, A.J. et al. Effects of barred owl (Strix varia) range expansion on Haemoproteus parasite assemblage dynamics and transmission in barred and northern spotted owls (Strix occidentalis caurina). Biol Invasions 17, 1713–1727 (2015). https://doi.org/10.1007/s10530-014-0828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0828-5