Abstract
Exotic Old World bluestem grasses (OWBG) are invading prairie and savanna ecosystems of the southern Great Plains USA, yet little is known about whether or how this invasion alters ecosystem processes. We conducted a study in a south Texas coastal prairie to address the following questions: (1) Does litter production, quality, decomposition rates, and soil nutrient dynamics differ significantly between areas dominated by OWBG versus native prairie?; and, (2) Does soil texture influence the effects of OWBG dominance on ecosystem processes? Ecosystem parameters were measured in adjacent patches of native and OWBG invaded coastal prairie on two soil textures, sandy loam and clay. Our findings indicate that ecosystem function differed between OWBG and native prairie dominated areas, but these results were not consistent across soil textures. On sandy loam soil, the soil microclimate differed substantially between OWBG and native prairie, and areas dominated by OWBG had higher aboveground plant production, soil organic C and total N pools, soil inorganic N concentrations and mineralization rates, and litter decomposition rates than native prairie. In contrast, on clay soils, these ecosystem properties often varied little between OWBG and native prairie. Dominance of OWBG appears to have altered native ecosystem function; yet, in this study the directionality and extent of these OWBG effects were strongly soil texture dependent, suggesting that local edaphic factors will likely interact with OWBG dominance in determining ecosystem properties. Consideration of these results may be valuable for managing OWBG such that control or restoration efforts on coarse-textured soils may be given priority over finer-textured soils.
Similar content being viewed by others
Introduction
Exotic species invasion, via deliberate introductions or inadvertent escapees, can result in unprecedented and sometimes seemingly irreversible environmental change and biodiversity loss (Chapin et al. 2000; Simberloff 1996; Vitousek et al. 1996). Exotic grass invasions, in particular, can result in large-scale and long-term ecosystem changes (Christian and Wilson 1999; D’Antonio and Vitousek 1992), and such invasions have been shown to alter nutrient cycling (DeMeester and Richter 2010; Rimer and Evans 2006), ecosystem N dynamics (Evans et al. 2001; Parker and Schimel 2010; Rossiter-Rachur et al. 2009), soil microbial communities (Hawkes et al. 2006; Kourtev et al. 2002), and litter decomposition dynamics (Allison and Vitousek 2004; Mack and D’Antonio 2003).
Globally, exotic grass invasions are a significant concern, as they can dramatically alter the ecosystem ecology of areas they invade (D’Antonio and Vitousek 1992; Mack 1989). For instance, cheatgrass (Bromus tectorum L.), an annual exotic grass, now occupies millions of acres of the Intermountain West, USA where it has invaded and spread throughout sagebrush—steppe eliciting widespread ecosystem effects (Evans et al. 2001; Morrow and Stahlman 1984; Stewart and Hull 1949). The conversion of perennial grass-dominated California grasslands to exotic annual grasses is another well-known example in which exotic grass invasions have had dramatic ecological impacts on a native ecosystem. Additional examples of highly invasive exotic grasses reported to alter ecosystem properties include: African lovegrasses (e.g., bufflegrass [Pennisetum ciliare]; Williams and Baruch 2000), cogongrass (Imperata cylindrica; Dozier et al. 1998), crested wheatgrass (Agropyron cristatum; Christian and Wilson 1999), gambagrass (Andropogon gayanus; Rossiter-Rachur et al. 2009), Japanese stiltgrass (Microstegium vimineum; DeMeester and Richter 2010), and saltmarsh cordgrass (Spartina alternifolia; Liao et al. 2008a).
The mechanisms and consequences of exotic plant invasions on ecosystem function have received a considerable amount of attention in the last decade (Bennett et al. 2011; Ehrenfeld 2003; Levine et al. 2003; Strayer et al. 2006). However, knowledge is generally lacking on how exotic plant invasions may alter ecosystem processes differently across the landscape depending on topoedaphic variability. It is well understood that soil texture affects soil nutrient dynamics (e.g., carbon and nitrogen mineralization) in many ecosystems (Hassinek 1992; Jenny 1980; Hook and Burke 2000), and in turn, plant species can also influence soil nutrient dynamics via alterations in primary production, litter quality, litter accumulation, and soil nutrient acquisition (Hobbie 1992; Wedin and Tilman 1990). Ehrenfeld (2003) emphasized that the effects of exotic plant invasions on nutrient cycling are not always “species-specific,” rather effects may often be site-specific. Thus, variation in soil conditions across the landscape may influence the magnitude of the effects of exotic plant invasions on ecosystem processes.
Here, we report underlying ecosystem differences between areas dominated by exotic Old World bluestem grasses (OWBG; Bothriochloa and Dichanthium spp.) and native C4 grasses on two contrasting soil types, sandy loam and clay, in a south Texas coastal prairie. OWBG are a group of perennial, C4 grasses indigenous to semi-arid and/or subtropical regions of southern Europe, Africa, Asia, and the Middle East (USDA, ARS 2011). OWBG were introduced to the central and southern Great Plains, USA in the early to mid-1900s (Celarier and Harlan 1955), and estimates suggest greater than one million ha of rangeland in Oklahoma and Texas have been seeded to OWBG since the mid-1980s for soil conservation and enhancement of rangeland forage and livestock production (McCoy et al. 1992; White and Dewald 1996). Traits such as greater seedling vigor, rapid developmental rates, greater productivity, and superior competitiveness over native congeners (i.e., Andropogon spp.) facilitated the adoption and widespread use of OWBG species (Coyne and Bradford 1985; Eck and Sims 1984; Harlan et al. 1958). Unfortunately, these same ‘superior’ traits may facilitate OWBG invasion and displacement of native C4 grass species (Schmidt et al. 2008).
Since the mid-twentieth century, King Ranch (KR) (Bothriochloa ischaemum var. songarica) and Kleberg (Dichanthium annulatum) bluestems have become increasingly abundant and problematic constituents of the rangelands in central and south Texas (Gabbard and Fowler 2007; Simmons et al. 2007) and throughout the entire central and southern Great Plains (Hickman et al. 2006; Reed et al. 2005). Populations of KR and Kleberg bluestems have displaced native species and threaten indigenous plant communities and biological diversity of the region (Gabbard and Fowler 2007). In the last decade or more, these species have invaded the coastal prairie region of south Texas. Coastal prairies, as a result of agricultural, commercial, and/or urban development, remain in only 1% of the 3.4 million ha originally present in pre-settlement Texas and Louisiana (Smeins et al. 1991). These invasive bluestem grasses are not the only species altering the ecosystem structure and function of these coastal prairies. Chinese tallow (Triadica sebifera, syn. Sapium sebiferum) invasions also significantly threaten the preservation of remnant Gulf Coast coastal prairies (Siemann and Rogers 2003) and have been shown to substantially alter nutrient cycling, species diversity, and herbivory interactions (Bruce et al. 1995; Rogers and Siemann 2002; Zuo et al. 2006). The combined pressures of Chinese tallow and OWBG invasions signify major challenges to the conservation of this highly threatened ecosystem (Diamond et al. 1992; Grossman et al. 1994).
To date, most investigations of OWBG have focused on testing various control-oriented management techniques, such as herbicide, prescribed fire, and/or disking (Harmoney et al. 2004; Mittlehauser et al. 2011; Simmons et al. 2007). A recent study determined that Andropogon bladhii (Caucasian bluestem; synonym Bothriochloa bladhii) invasion of a tallgrass prairie reduced C and N cycling and plant diversity (Reed et al. 2005). However, little is known regarding the effects of OWBG invasions on other grassland ecosystem functions or if these effects are consistent across differing soil textures. Therefore, we conducted a study in a south Texas coastal prairie where OWBG are invading and presently co-exist with native prairie in a mosaic-type landscape pattern on two contrasting soil textures, sandy loam and clay. The objective of this study was to determine if underlying ecosystem function differences existed between OWBG dominated and native coastal prairie, and if so, were these differences consistent across both sandy loam and clay soils. We asked the following questions: (1) Does litter production, quality, decomposition rates, and soil nutrient dynamics differ in areas invaded by OWBG from that observed in adjacent native prairie?; and (2) Are the effects of OWBG dominance in coastal prairie ecosystem processes independent of soil texture? Based on visually apparent differences in canopy cover and litter accumulation between the two vegetation types on both soil types, we hypothesized that OWBG invasion and dominance in this native coastal prairie has altered plant–soil interactions and ecosystem processes. Furthermore, on-site observations indicating OWBG are similarly invasive on both sandy loam and clay soil types led us to predict that OWBG dominance would have consistent effects on ecosystem function throughout the landscape where it occurs.
Methods
Site description
Research was conducted at the Rob and Bessie Welder Wildlife Foundation Refuge in San Patricio County, Texas (28°07′ N, 97°24′W), approximately 19 km north of Corpus Christi. The Welder Refuge encompasses 3,120 ha and is located in a transitional area between the Gulf Coast Prairies and Marshes and the South Texas Plains Ecoregions (Gould 1962). Relative humidity of this region is high due to the proximity to the Gulf of Mexico, but the climate is variable and often extreme with periods of above and below average temperature and rainfall. The region is generally classified as having a subtropical and semi-arid climate (Drawe et al. 1978; Scrifres and Hamilton 1993). Long-term (1971–2000) mean annual temperature, daily maximum, and daily minimum temperatures at the Welder Refuge are 21.3, 26.8, and 15.8°C, respectively (NOAA 2010). Long-term mean annual precipitation from 1956 to 2008 was 923 mm (T. Blankenship, Welder Wildlife Foundation), and annual precipitation amounts during the study period were 1,259 mm (2007), 450 mm (2008), and 738 mm (2009). Tropical storm activity in 2007 led to July and August rainfall totals of 549 and 128 mm in that year (729 and 156% greater than the long-term norms, respectively).
Clay and clay loam soils, derived from Late Pleistocene marine sediments, are the predominant soil type on the Welder Refuge, but alluvial sandy loams are common near riparian, depositional zones (Drawe et al. 1978; Soil Survey Staff, NRCS, USDA 2010). OWBG have been increasing in dominance in the native coastal prairie on the Welder Refuge over the last decade or more (Drawe DL, and Blankenship T, pers. comm.), such that the remaining prairie has been largely transformed into a mosaic of native vegetation and exotic OWBG patches. Patches and/or stands of OWBG range in size from a few established plants (<0.5 m in cover) to greater than two ha in size, and are co-dominated by Kleberg (Dichanthium annulatum) and KR bluestems (Bothriochloa ischaemum var. songarica) on both sandy loam and clay soils. Patches of native coastal prairie are dominated by little bluestem (Schizachyrium scoparium; Drawe et al. 1978) on both sandy loam and clay soils; other native grasses (Hatch et al. 1999) and forb species generally comprise ≤20% of the total plant canopy cover. While we do not know the exact length of time any particular stand of OWBG invaded coastal prairie has existed, we estimate that most have been established for at least 8–10 years.
Fire has long been reported as an important natural disturbance for maintenance of the native grasslands in south Texas (Scrifres and Hamilton 1993). Dormant (late winter/early spring) and growing season prescribed burns have been conducted at Welder Refuge for decades to manage the plant communities, principally with regards to control of woody plants and to maintain vigor of the native coastal prairie. Under favorable conditions, prescribed burns are conducted on specific units of the Welder Refuge every 3 years; however, unfavorable weather and fuel load conditions may result in some units being burned on longer rotations.
Experimental design
We identified 5 m × 5 m paired plots of native coastal prairie (hereafter native grasses) and exotic OWBG stands (n = 6 of each vegetation stand type) on sandy loam (Odem fine sandy loam and Delfino loamy fine sand) and clay (Victoria clay) soils (Soil Survey Staff, NRCS, USDA 2010). Each of the paired plots constituted a block (i.e., replicate) of each vegetation type on each soil type (total n = 24; Fig. 1). Paired plots of OWBG and native grasses were dominated by Kleberg bluestem (>80% canopy cover) and little bluestem (≥70% canopy cover) (data not shown), respectively, and they generally did not differ in soil % clay, bulk density, or pH for either soil type. However, these parameters differed dramatically between the two soils types (Appendix 1 in ESm). All plots were located ≥10 m from woody vegetation to minimize any woody plant influence on grassland soil nutrient dynamics. Dormant season burns were conducted in 2003, 2005, and 2008 on the unit where we located the paired native grass and OWBG plots on the clay soil, but the unit in which the sandy loam paired plots were located had not been burned since 1997 (T. Blankenship, Welder Wildlife Foundation, unpublished records).
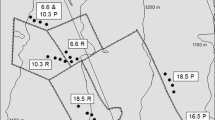
Location within Texas (as indicated by star) and soil map of the Rob and Bessie Welder Wildlife Foundation Refuge (a) in San Patricio County, Texas, USA. b represents an enlargement of the areas in which 5 m × 5 m paired plots of native grasses (open triangles) and Old World bluestem grasses (filled triangles) were located on sandy loam and Victoria clay soils, respectively. † Od Odem fine sandy loam, Delfino Delfino loamy fine sand
Soil microclimate
Soil temperature and moisture were measured at random locations within each plot. Soil temperatures were measured weekly at 5 cm depth with a temperature probe at mid-day, between 1200 and 1400 h, during the 2008 growing season (26 May–19 September). Soil moisture (0–10 and 10–20 cm depth increments) was determined gravimetrically on subsamples (10 g) obtained from soil cores taken for inorganic N analysis (see section below). Weighed subsamples were oven-dried at 105°C for 48 h and then re-weighed. Volumetric soil water content (cm3 H20 cm−3 dry soil) was calculated by multiplying the gravimetric soil moisture by the soil bulk density.
Soil sampling and nutrient dynamics
Soil samples (0–20 cm) were collected from random locations within each plot using a slide hammer soil core (i.d. 4.8 cm; AMS, Inc., American Falls, Idaho, USA). Soil samples were collected periodically during the 2007 active growing season (12 and 20 June 2007; 02 and 08 August 2007), post-peak of the 2007 growing season (25 October 2007), prior to the onset of the 2008 active growing season (16 January and 22 February 2008), and lastly during the 2008 active growing season (25 May 2008 and 05 June 2008). At every soil sample collection period, each 0-20 cm soil core was divided into 0–10 and 10–20 cm depth increments, placed in separate air-tight plastic collection bags, and put on ice in a cooler for transport to the laboratory. Soil cores were kept refrigerated at approximately 4°C in the laboratory until further soil processing could occur (≤5 days). Sandy loam soil samples were sieved through a 2 mm sieve and all visible roots, aboveground plant material, and rocks were removed by hand. Due to the high clay content (45–54%) of the clay soils, sieving was not possible, and instead they were homogenized by hand-mixing and all visible roots, aboveground plant material, and rocks removed. Soil inorganic N (Ninorg; NH4 + and NO3 −) was extracted with 2 M KCl (50 ml 2 M KCl: 25 g processed soil sample) (Maynard and Kalra 1993) and analyzed for Ninorg using an automated colorimetric technique (Rice et al. 1984).
In situ net nitrogen mineralization (Nmin) rates were measured in each vegetation type in both sandy loam and clay soils using field incubations and ion-exchange resin capsules (Lanxess Corporation, Sybron Chemicals, Inc., Birmingham, NJ) from 24 May to 24 June 2008. Resin capsule bags were constructed from nylon stocking and contained a mixture of equal proportions (10 g) of cation (Ionac C-267) and anion (Ionac ASB-1P) capsules. Two soil cores (4.8 cm diameter × 15.1 cm long) were extracted with a slide hammer directly adjacent to each other in each plot. One core was immediately placed in an air-tight plastic collection bag and put on ice in a cooler for transport to the laboratory for initial measurements of Ninorg. The second core was kept in the aluminum core sleeve used during sample collection, resin capsule bags were placed at the bottom of each core, and the core was returned to the hole in the orientation from which it was extracted (Raison et al. 1987). At the end of the 30-day in situ incubation period, the cores containing soil and resin capsule bags were extracted and the concentrations of Ninorg were determined as described previously. In situ net Nmin rates were calculated as the difference between the initial and final soil total Ninorg plus the resin bead total Ninorg divided by the in situ incubation period (Raison et al. 1987). Negative values indicated N was immobilized by microbes during the incubation and positive values indicate N was mineralized and released.
An air-dried subsample (≈100 g) from soil cores taken 20 June 2007 from each plot for soil Ninorg was used for soil physicochemical analysis (both soil depths). These analyses were performed by the University of Kentucky’s Division of Regulatory Services (http://soils.rs.uky.edu/tests/methods.php#Routine). Each subsample was tested for pH, soil texture (% sand, silt, and clay; using the micropipette method), and concentrations of phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) using the Mehlich III extraction (Soil and Plant Analysis Council 2000). Soil organic C (SOC) and total N pools (g m−2) were also determined from subsamples of the 20 June 2007 soil cores. These subsamples were ball-mill ground and oven dried at 55°C for 24 h prior to analysis. Subsamples were acid fumigated to remove inorganic C prior to SOC analysis (Harris et al. 2001). Percent C and N were analyzed with a Flash Elemental Analyzer 1112 (Thermo Fisher Scientific Inc., Waltham, MA).
Additional soil cores (4.8 cm diameter × 15.1 cm depth) collected 20 June 2007 were used to determine soil bulk density from each plot. Soil bulk density (g cm−3) was calculated by dividing the weight of the root and rock-free oven-dried (105°C for 48 h) soil by the volume extracted. These bulk density values were used to convert soil nutrient concentrations to pool sizes.
Litter decomposition
Senesced native and OWBG litter was collected within 5 m from experimental plots on each soil type (2 composite litter types (native grass and OWBG) × 2 soil types = 4 litter samples) on 18 January 2007. Each litter sample was manually cut into 4–6 cm pieces, well mixed, and oven-dried at 55°C for 48 h. Approximately 5 g of oven-dried litter was placed into each litterbag (10 cm × 10 cm) constructed of fiberglass mesh screening with 1.5 mm2 openings. During handling and transport of litterbags to the field site, a small amount of litter was lost from each litterbag; therefore, the t = 0 weight for each litter type was adjusted accordingly. The average mass of OWBG litterbags at t = 0 from both sandy loam and clay soil was 4.94 ± 0.01 g, while native grass litter at t = 0 averaged 4.88 ± 0.0 and 4.95 ± 0.01 g from the sandy loam and clay soils, respectively.
A soil type-specific litterbag experiment was conducted whereby OWBG and native grass litter was incubated reciprocally in both OWBG invaded and native coastal prairie plots, yet only on the soil type in which the litter originated. Six litterbags, corresponding to six planned collection dates, of each litter type (n = 12 litterbags per plot) were randomly placed in each replicate vegetation stand type (i.e., OWBG or native grass) on each soil type on 22 February 2007 (total n = 144 litterbags). Litterbags were collected from the field at 0, 14, 30, 90, 195, and 455 days. At each collection period, litterbags were air-dried for several days, and oven dried at 55°C for 48 h. After drying, litter was weighed and ground in a cyclone grinder, and a subsample was further ball-ground. Subsamples of the ball-ground material were ashed in an oven at 550°C for 6 h to determine proportion of soil contamination in litter samples. Litter remaining at each time point was calculated on an ash—and soil—free basis (Blair 1988). Litter mass loss (mt/m0) over the study period (Feb 2007 to May 2008) was used to calculate litter decomposition rates, k (year−1), by fitting the following equation to the data from each replicate patch for each litter type (Olson 1963):
Percent C and N of each litter sample were determined by combusting a ~25 mg subsample in a Flash Elemental Analyzer 1112. The mass of N (mg) within each litter sample was calculated by multiplying litter %N by the ash- and soil-corrected litter dry mass. Litter N retention was calculated by dividing the litter N mass (mg) of each litter sample at each collection time period (Nt) by the initial litter N mass at day zero (Nt/N0).
Aboveground net primary production (ANPP)
We measured ANPP in each plot in 2007 and 2008 by harvesting plant biomass at standing peak production which generally occurs from August through October at this site. In 2007, plant biomass was clipped at ground level from five 0.125 m2 quadrats (subsamples) randomly placed within each replicate plot on August 12, and in 2008, plant biomass was harvested from three 0.125 m2 quadrats per plot on October 14. Each biomass subsample was sorted into previous year’s standing dead grasses and forbs (discarded), present year standing dead forb and grasses, and standing live grasses and forbs. ANPP is the sum of forb and grass aboveground biomass (including live and present year standing dead). Each biomass sample was dried at 55°C for 48 h, weighed, ground in a Wiley Mill, and then ball-mill ground. Since total ANPP was generally >95% grass biomass, %C and %N was only determined from grass samples.
Statistical analyses
We analyzed all datasets with SAS® 9.1 statistical software (SAS® Institute Inc., Cary, NC). A factorial repeated measures mixed model analysis (PROC MIXED) was performed on soil temperature, soil moisture, and soil Ninorg (Littell et al. 1996). Fixed effects were vegetation stand type, soil type, and sample collection time; block (soil) was the random effect; and time was the repeated measure. To assess the effects of vegetation stand type and litter type on litter mass loss, a repeated measures mixed model was utilized using vegetation stand type (native or OWBG), litter type (native or OWBG), soil type, and time (i.e. number of days of litterbag incubation) as the fixed effects, block (vegetation stand type × litter type) as the random effect, and time as the repeated measure.
For parameters not measured repeatedly over time, we used a factorial mixed model analysis (PROC MIXED) without the repeated component. These parameters included net Nmin, ANPP and associated parameters (% N, aboveground N pools and C:N), litter decomposition rate (k), initial litter chemistry, and soil physicochemical data. ANPP data were analyzed on a ‘by year’ basis because biomass harvests in 2007 and 2008 occurred at different times during standing peak production. In these analyses, we examined the fixed effects of vegetation stand type and soil type and their interaction with block (soil) as the random effect.
All possible main effects and interactions were included in all models. We examined all response variables for normality and equality of variance, and if needed, transformations were performed on the response variables that did not meet these two assumptions for ANOVA. Post hoc least-squared means (LSMEANS) Tukey-adjusted mean comparison tests were performed in all analyses when main effects were P ≤ 0.05.
Results
Effects on soil microclimate
Highly significant vegetation * soil interaction effects (P < 0.0001) for both soil temperature and moisture indicated vegetation stand type had a strong effect on soil microclimate that depended on soil texture (Appendix 2 in ESM; Figs. 2, 3). On sandy loam soil, soil temperatures were often 2–4°C cooler in areas dominated by OWBG compared to native grasses (Fig. 2a); however, this trend reversed on clay soil (i.e., soil temperatures in native grass were cooler than OWBG; P = 0.01; Fig. 2b). Soil moisture at 0–10 cm was higher in OWBG than adjacent natives on sandy loam soils (P < 0.0001), but it did not differ between vegetation stand types on the clay soil (Fig. 3). At 10–20 cm, soil moisture was higher under OWBG compared to native grasses on both sandy loam and clay soil (P < 0.0001; Fig. 3). In addition, soil temperature and moisture varied temporally by vegetation and soil type (Appendix 2 in ESM).
Effects on soil nutrient pools and dynamics
Soil organic carbon pools (SOC) did not differ (P > 0.05) between vegetation stand types at the 0–10 cm depth increment on either soil type, but at 10–20 cm depth, areas dominated by OWBG had greater SOC pools than native grasses on both clay and sandy loam soils (P = 0.008; Table 1). The effect of OWBG dominance on SOC pools at 10-20 cm was strongest on the sandy loam soil, yet no veg * soil interaction was detected (P = 0.15). When both depth increments were summed, SOC pools of sandy loam soil were 67% greater in areas dominated by OWBG than adjacent natives (veg * soil P = 0.05; Tukey-adjusted test, P = 0.02), but there was no difference between vegetation stand types in the top 20 cm on the clay soil (Tukey-adjusted test, P = 0.98) .
Similar to SOC pools, there was no difference between OWBG and native grasses in total soil N pools at 0–10 cm (P = 0.49; Table 1). However, at the 10–20 cm depth increment, there was a veg * soil interaction (P = 0.04), reflecting greater total N pools under OWBG on sandy loam soils only (3× greater N in 10–20 cm under OWBG than native grasses).
These trends in SOC and total N pools resulted in differences in soil C:N ratios between OWBG and native grasses. While at the 0–10 cm depth, soil C:N was similar between vegetation stand types and across both soil types, at the 10–20 cm depth and the 0–20 cm total, OWBG had lower soil C:N ratios than native grasses on the sandy loam, but not the clay soil (veg * soil: P = 0.01; Table 1).
Soil pools of potassium were greater under OWBG compared to adjacent native grasses at all depth increments on both sandy loam and clay soil (Table 1). Soil pools of magnesium were also elevated under OWBG compared to adjacent native grasses on both soil textures but only in the 0–10 cm depth increment (Table 1). Soil pools of phosphorus or calcium did not differ between vegetation stand types, although all soil macronutrient pools differed between soil types (Table 1).
Soil Ninorg did not vary between vegetation types at the 0–10 cm or 10–20 cm soil depths (Appendix 3 in ESM; Fig. 4a–d). This finding was consistent for concentrations of soil NH4 +–N and NO3 −–N when analyzed independently (data not shown). However, veg * soil type interactions were detected for both 0–10 cm (P = 0.0004) and 10–20 cm (P = 0.046) depths (Appendix 3 in ESM). Total soil Ninorg was higher (albeit marginally significant P = 0.06) under OWBG compared to adjacent native grasses at 0–10 cm on sandy loam (Fig. 4a), yet the reverse trend was observed in clay soil: total soil Ninorg was greater under native grasses compared to OWBG (P = 0.05) (Fig. 4b). Tukey-adjusted mean comparisons failed to detect a significant effect of vegetation stand type on total soil Ninorg at 10–20 cm on either soil texture (Fig. 4c, d). Significant temporal variations in total soil Ninorg concentrations also were evident, but non-significant veg * time and veg * soil * time interactions suggest that OWBG and native grass dominated areas have similar total soil Ninorg temporal patterns but these patterns differ between the two soil types (Fig. 4a–d; Appendix 3 in ESM).
Total in situ net Nmin rates differed between vegetation and soil types, and the veg * soil interaction was non-significant (Fig. 5). N immobilization was the dominant process under native grasses across both soil types, and while N also was immobilized under OWBG on the clay soil, the rate was substantially less than that of adjacent native grasses (Fig. 5). On the sandy loam soil, N was mineralized under OWBG. Overall, net Nmin rates were higher in sandy loam versus clay soils and higher for OWBG versus native grasses (Fig. 5).
Effects on litter decomposition
Litter mass loss differed by litter type and across time (Appendix 4 in ESM), with OWBG litter decomposing almost 2× faster than native grass litter on both soil types, but litter mass loss did not differ by vegetation stand type or soil type (Fig. 6a, b; Appendix 4 in ESM). These results suggest OWBG litter was more labile than native grass litter, which is supported by measured initial litter C:N and lignin:N ratios (Table 2). Temporal patterns of mass loss varied between soil types which led to a significant soil type effect on overall litter decomposition rates (k; P = 0.006): litter (both OWBG and native) decomposed more quickly on sandy loam soils (Table 2). The temporal patterns of mass loss varied between the two soil types (soil * time P = 0.0005; Appendix 4 in ESM), and a significant three way interaction between vegetation stand type * soil * time was primarily the result of OWBG and native grass litters decomposing differently when incubated in the reciprocal vegetation stand type on the sandy loam soils only (Fig. 6a, b).
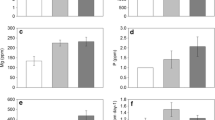
Litter mass loss (mt/m0) (a, b), C:N (c, d), and N retention (e, f) of native and Old World Bluestem grass (OWBG) litter incubated in native and OWBG vegetation stand types on sandy loam (a, c, e) and clay soil (b, d, f). Bars represent mean ± 1 SE. Note In the figure legend, the first word (‘native’ or ‘OWBG’) indicates the vegetation stand type where the corresponding litter was incubated
Litter C:N ratios over the decomposition study were impacted by soil type, litter type, time, and vegetation stand type (Appendix 4 in ESM). Significant veg * soil, soil * litter, litter * time, and veg * soil * litter interactions reflect that temporal patterns of litter C:N for native and OWBG litters incubated in their reciprocal vegetation stand type differed between the two soil types. In clay soils, native and OWBG litter behaved similarly with regard to changes in C:N regardless of which vegetation stand type they were incubated: native grass litter always had a significantly higher C:N than OWB grass litter (Fig. 6d). However, in sandy loam soils, native grass litter experienced more substantial decreases in C:N when incubated in OWBG compared to native grasses, and OWBG litter incubated in native grasses maintained higher C:N ratios than when it was incubated in OWBG stand types (Fig. 6c).
Patterns of litter N retention also differed between vegetation stand type, soil type, litter type, and time (Appendix 4 in ESM). Significant soil * time, litter * time, and veg * time two-way interactions were also identified. In general, litter decomposing in clay soils tended to retain more N than in sandy loam soils, although this effect was only significant, according to post hoc Tukey-adjusted mean comparisons, at the 90 day collection period and beyond (Fig. 6e, f). By the later collection periods, native grass litter retained significantly more N than OWBG litter. The veg * time interaction indicated that when averaged across litter types and soil types different N retention patterns were observed for the OWBG and native grass vegetation stand types. Post hoc Tukey-adjusted mean comparisons suggested litter N retention differed between vegetation stand types: it tended to be higher in native grass versus OWBG stands, but this effect was only significant at the 90 day litterbag collection time point (P = 0.006; Fig. 6e, f). A non-significant veg * litter * soil interaction suggested that these effects of OWBG invasion on litter N retention were not soil type dependent.
Effects on ANPP and associated parameters
In 2007, areas dominated by OWBG had greater aboveground N pools than adjacent native grass stands on both sandy loam and clay soils (P = 0.04), yet ANPP and other associated parameters were similar between vegetation types (Table 3; Appendix 5 in ESM). Sandy loam soils had more plant available N than clay soils, as indicated by both native and OWBG having greater %NANPP, aboveground N pools, and lower C:NANPP (Table 3). Marginally significant veg * soil interactions for ANPP (P = 0.065; Appendix 5 in ESM) and aboveground N pools (P = 0.08) reflect that differences between native and OWBG tended to be strongest on sandy loam soils (Table 3).
In contrast to 2007, the 2008 biomass harvest showed no soil type differences in %NANPP or C:NANPP; however, strong differences between vegetation stand types were observed in these parameters (both P < 0.0001; Appendix 5 in ESM). OWBG had lower %NANPP and higher C:NANPP than adjacent native grass stands; however, because ANPP tended to be greater in OWBG compared to native grasses on both soil types (P = 0.06), similar aboveground N pools between vegetation types was observed (Table 3). Greater ANPP for both native and OWBG was measured on clay soils during this year (P = 0.004; Table 3), and since no difference between soil types was observed for %NANPP, these production trends led to higher aboveground N pools also being measured on clay versus sandy loam soils.
Discussion
Conversion of Texas coastal prairie from dominance by native grasses to that of exotic OWBG is likely to alter ecosystem function (Table 4). For example, OWBG litter decomposed faster and released more N than native grass litter regardless of where it was incubated in the litter decomposition experiment. Similarly, OWBG dominated areas had increased soil potassium and magnesium pools and tended to immobilize less N than native grass patches on both soil types. These results support recent studies which have shown that exotic plant invasions generally accelerate litter decomposition and nutrient cycling rates (Allison and Vitousek 2004; Ehrenfeld 2003; Liao et al. 2008b). However, we also found that the effects of OWBG invasion on some ecosystem parameters were contingent on soil type.
Soil physicochemical properties directly affect soil nutrient storage and availability (Hassinek 1992; Jenny 1980). In general, carbon and nitrogen mineralization is more rapid in sandy versus clay soils (Hassinek 1992); however, for this study, because observations suggested OWBG are equally invasive on sandy and clay soils, we hypothesized that inherent soil property differences would have little impact on the effects of OWBG dominance on grassland ecosystem function. However, this hypothesis was not substantiated for several measured ecosystem parameters, as we measured more subtle or opposite effects of OWBG dominance on the clay compared to the sandy loam soils. For example, soil moisture was higher, and soil temperatures were lower in surface soils under OWBG relative to native prairie on sandy loam soils, but these patterns were either not significant or reversed between vegetation types on the clay soils (Table 4). These soil microclimate effects could be the result of vegetation type differences in water use efficiency or plant cover, litter production and accumulation effects on water loss via evapotranspiration. It is likely that such soil microclimate differences may account for many of the observed differences in soil nutrient availability and cycling. For example, total soil Ninorg tended to be higher under OWBG than native prairie in surface sandy loam soils, but total soil Ninorg was significantly lower under OWBG than native prairie at the same depth increment in clay. While SOC pools tended to be higher under OWBG on both soil types, the SOC differences between OWBG and native prairie were much larger on sandy loam soils (Tables 1, 4). Soil C:N ratios also were lower under OWBG on sandy loam, yet on clay soil, soil C:N ratios did not vary significantly between vegetation types. These results combined with the significant differences observed in plant production, litter chemistry and decomposition patterns suggest that ecosystem processes are altered with OWBG dominance in this coastal prairie ecosystem, but not always in a consistent manner across the landscape. Further research evaluating the causes and consequences of the altered soil microclimate accompanying OWBG dominance in these landscapes on ecosystem function seems warranted.
Reed et al. (2005) also found that the effects of OWBG invasion can vary across the landscape; however, in that case, it was differences in fire regime, not soil texture, which explained the variation. Reed et al. (2005) determined that areas of tallgrass prairie in Kansas invaded by Andropogon bladhii (Caucasian bluestem) had lower soil %C and %N than adjacent native grass (A. gerardii) stands but only in frequently burned areas (i.e., soil %C and N were similar in unburned OWBG and native grass stands). Many of the OWBG effects observed by Reed et al. (2005) in tallgrass prairie were opposite in nature to those measured in this study. For example, Reed et al. (2005) measured reduced soil C pools and lower N mineralization rates in OWBG invaded areas: we observed increases for both parameters. Reed et al. (2005) concluded that prescribed burning, which is utilized to maintain tallgrass prairie, may likely interact with A. bladhii invasion to have long-term effects on ecosystem fertility. Although fire is also employed at our study site, it was beyond the scope of this study to investigate the interactions of fire, soil texture, and OWBG invasion on ecosystem processes. It would be interesting to determine whether fire strengthened, reversed, or had no interaction with the invasive grass effects on ecosystem processes at this site. The results of Reed et al. (2005) and those from this study demonstrate how closely related exotic grass species can have variable effects on ecosystem processes, and how these effects may be highly contingent on site-specific events and/or characteristics such as disturbance (i.e., burning) and soil texture (Ehrenfeld 2003). Such contingencies make understanding or predicting the effects of invasive species at large geographic scales especially challenging.
Complex interacting factors, such as disturbance regime, propagule pressure, and edaphic and weather conditions influence the ability of exotic species to invade particular community or ecosystem types and the subsequent rate of invasion (Thébaud et al. 1996; Lambrinos 2002). Many of these factors also vary over time. The 2 years of our study experienced dramatically different rainfall regimes (>800 mm more rainfall was delivered at the site in 2007 than 2008), and undoubtedly, the clay and sandy loam soils interacted with this rainfall to produce some of the variation that we observed. For example, ANPP was higher in OWBG plots on sandy loam soils only in 2007, the wet year, which may have been associated with the fact that these soils drain better than the clay soils: standing water was present on the clay soils at times during the 2007 growing season. Perhaps, OWBG are more productive and thus, more competitive than native grass species only under well-drained or water-limited conditions. Rainfall was low in 2008 (450 mm), and OWBG had greater ANPP than native grass stands on both soil types that year.
Additional evidence for complex interactions occurring between plant species, soil type, and time exists in both the litter decomposition experiment and the tissue chemistry of annual plant production dataset. Although when viewed across the whole litter decomposition experiment, effects of OWBG were relatively consistent across soil types in stimulating mass loss and reducing N retention (Table 4), a more detailed examination of the numerous interactions between vegetation stand type, litter and soil types, and time in the complete litter decomposition dataset clearly indicate subtle variation in these ecosystem response parameters across the landscape and over time (Fig. 6; Appendix 4 in ESM). In addition, we also observed that trends in aboveground production litter chemistry between the two grass types (native and OWBG) depended on when the litter was harvested. For example, the mid-August 2007 biomass harvest showed no significant difference in OWBG or native grass material in %N or C:N in either sandy loam or clay soils (though there were differences between soil types). However, the mid-October 2008 biomass harvest indicated OWBG had significantly less %N and higher C:N ratios than native grasses on both sandy loam and clay soils, and then, in January 2008, the litter collected for the litter decomposition experiment showed just the opposite: OWBG had higher %N and significantly lower C:N ratios than native grass litter. These results suggest that there are species-specific differences in the timing of re-translocation and the quantity of N transferred at the end of the growing season from above- to belowground tissue between these perennial grasses (i.e., native grasses may re-translocate more N but do so later in the year than exotic OWBG). Whether such temporal changes in the N contained in aboveground plant material resulting from the replacement of native species with exotics has repercussions for herbivores or ecosystem processes remains to be evaluated. Work with another prominent invasive species in these coastal prairie systems, Chinese tallow, clearly shows significant links between invasive species dominance and arthropod diversity and damage from herbivory (Hartley et al. 2010).
OWBG invasions pose a significant challenge to the conservation and restoration of native prairies and savannas of the central and southern Great Plains, USA (Gabbard and Fowler 2007; Mittlehauser et al. 2011; Simmons et al. 2007). Our results show a strong correlation between OWBG dominance and altered ecosystem function; however, because we were unable to measure these parameters at these sites before OWBG invasion and dominance, we cannot rule out the possibility that there were pre-existing, microsite differences across our stands. However, given that each block of OWBG and native prairie plots were paired adjacent to each other (i.e., within several meters) and replicated six times across the soil type, that no visible microsite topographical or geologic differences are known to exist within the soil types, and that there were no differences in all other basic soil parameters (texture, pH, or bulk density) between paired vegetation stand type blocks, we think it is unlikely that such pre-existing microsite differences account for our results. Pre-invasion measurements, specifically focused on soil nutrient and microclimatic parameters, would greatly strengthen our understanding of both the causes and consequences of OWBG dominance in this system.
This study highlights an interesting pattern of an exotic plant invasion—the directionality and degree of OWBG invasion effects on ecosystem function was highly dependent on edaphic conditions (soil texture). This conditionality of effects suggests that OWBG invasion may impact certain areas more than others (e.g., at this site, sandy loam soils may be more sensitive to OWBG induced changes than clay soils). While these findings warrant further evaluation across multiple study sites, they suggest that edaphic conditions should be considered and may be valuable for managing OWBG such that control or restoration effects on coarse-textured soils are given priority over finer-textured soils.
References
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants from Hawaii. Oecologia 141:612–619
Bennett AE, Thomsen M, Strauss SY (2011) Multiple mechanisms enable invasive species to suppress native species. Am J Bot 98:1086–1094
Blair JM (1988) Nitrogen, sulfur, and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians. Soil Biol Biochem 20:693–701
Bruce KA, Cameron GN, Harcombe PA (1995) Initiation of a new woodland type on the Texas coastal prairie by the Chinese tallow tree (Sapium sebiferum (L.) Roxb.). Bull Torrey Bot Club 122:215–225
Celarier RP, Harlan JR (1955) Studies on Old World bluestems. Ok Agric Exp Station Tech Bull, T-58
Chapin FS III, Zaveleta ES et al (2000) Consequences of changing biodiversity. Nature 405:234–242
Christian JM, Wilson SD (1999) Long-term ecosystem impacts of an introduced grass in the northern Great Plains. Ecology 80:2397–2407
Coyne PI, Bradford JA (1985) Morphology and growth in seedlings of several C4, perennial grasses. J Range Manag 38:504–512
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
DeMeester JE, Richter DdeB (2010) Differences in wetland nitrogen cycling between an invasive grass Microstegium vimineum and a diverse plant community. Ecol Appl 20:609–619
Diamond D, Amon B, Cook T, Edwards R, Elliot W, Evans R, Hayes T, Kennedy K (1992) Endangered, threatened, and watch list of natural communities of Texas. Texas Organ Endanger Species, Austin
Dozier H, Gaffney JF et al (1998) Cogongrass in the United States: history, ecology, impacts, and management. Weed Technol 12:737–743
Drawe DL, Chamrad AD, Box TW (1978) Plant communities of the Welder Wildlife Refuge. Contribution No. 5, Series B, Revised. Welder Wildl Found, Sinton
Eck HV, Sims PL (1984) Grass species adaptability in the Southern High Plains—a 36-year assessment. J Range Manag 37:211–217
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Evans RD, Rimer R, Sperry L, Belnap J (2001) Exotic grass invasion alters nutrient dynamics in an arid grassland. Ecol Appl 11:1301–1310
Gabbard BL, Fowler NL (2007) Wide ecological amplitude of a diversity-reducing invasive grass. Biol Invasions 9:149–160
Gould FW (1962) Texas plants—a checklist and ecological summary. Texas Agric Exp Station, MP-585
Grossman DH, Goodin KL, Reuss CL (1994) Rare plant communities of the conterminous United States. The Nature Conservancy, Arlington
Harlan JR, Celarier RP, Richardson WL, Brooks MH, Mehra KL (1958) Studies on Old World bluestems II. Ok Agric Exp Station Tech Bull, T-72
Harmoney KR, Stahlman PW, Hickman KR (2004) Herbicide effects on established yellow Old World Bluestem (Bothriochloaischaemum). Weed Technol 18:545–550
Harris D, Horwath WR, van Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci Soc Am J 65:1853–1856
Hartley MK, Rogers WE, Siemann E (2010) Comparisons of arthropod assemblages on an invasive and native trees: abundance, diversity, and damage. Arthropod-Plant Interact 4:237–245
Hassinek J (1992) Effects of soil texture and structure on carbon and nitrogen mineralization in grassland soils. Biol Fertil Soils 14:126–134
Hatch SL, Schuster JL, Drawe DL (1999) Grasses of the Texas Gulf Prairies and Marshes. W.L. Moody, Jr. Natural History Series, no. 24, Texas A&M Univ Press, College Station
Hawkes CV, Belnap J, D’Antonio CM, Firestone MK (2006) Arbuscularmycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant Soil 281:369–380
Hickman KR, Farley GH, Channell R, Steirer JE (2006) Effects of Old World Bluestem (Bothriochloa ischaemum) on food availability and avian community composition within the mixed-grass prairie. Southwest Nat 51:524–530
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Hook PB, Burke IC (2000) Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate. Ecology 81:2686–2703
Jenny H (1980) The soil resource: origin and behavior. Springer, New York
Kourtev PS, Ehrenfield JG, Häggblom M (2002) Exotic plant species alter microbial community structure and function in the soil. Ecology 83:3152–3166
Lambrinos JG (2002) The variable invasive success of Cortaderia species in a complex landscape. Ecology 83:518–529
Levine JM, D’Antonio CM, Dukes JS, Grigulus K, Lavorel S, Vila M (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
Liao CZ, Luo YQ, Fang CM, Chen JK, Li B (2008a) Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze Estuary. Oecologia 156:589–600
Liao C, Ping R, Zhou X, Wu X, Bo L (2008b) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute Inc., Cary
Mack RN (1989) Temperate grasslands vulnerable to plant invasion: characteristics and consequences. In: Drake JA, Mooney HA et al (eds) Biological invasions: a global perspective. Wiley, New York, pp 155–179
Mack MC, D’Antonio CM (2003) The effects of exotic grasses on litter decomposition in a Hawaiian woodland: the importance of indirect effects. Ecosystems 6:723–738
Maynard DG, Kalra YP (1993) Nitrate and exchangeable ammonium nitrogen. In: Carter MR (ed) Soil sampling methods and analysis. Lewis, Boca Raton, p 25
McCoy SD, Mobley JC, Engle DM (1992) Old World bluestem seedings in western Oklahoma. Rangelands 14:41–44
Mittlehauser JR, Barnes PW, Barnes TG (2011) The effect of herbicide on the re-establishment of native grasses in the Blackland prairie. Nat Areas J 31:226–233
Morrow LA, Stahlman PH (1984) History and distribution of downy brome (Bromus tectorum) in North America. Weed Sci 32(Suppl 1):2–6
National Oceanic and Atmospheric Administration (NOAA) (2010) Climate normals, 1971–2000 NCDC monthly normals. NOAA Southern Regional Climate Center, Louisiana State University, Baton Rouge. www. srcc.lsu.edu/climate Normals/. Accessed 8 Jan 2010
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Parker SS, Schimel JP (2010) Invasive grasses increase nitrogen availability in California grassland soils. Invasive Plant Sci Manag 3:40–47
Raison RJ, Connell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral-N in situ. Soil Biol Biochem 19:521–530
Reed HE, Seastedt TR, Blair JM (2005) Ecological consequence of C4 grass invasion: a dilemma for management. Ecol Appl 15:1560–1569
Rice CW, Smith MS, Crutchfield JM (1984) Inorganic N analysis of soil extracts by automated and distillation procedures. Commun Soil Sci Plant Anal 15:663–672
Rimer RL, Evans RD (2006) Invasion of downy brome (Bromus tectorum L.) causes rapid changes in the nitrogen cycle. Am Midl Nat 156:252–258
Rogers WE, Siemann E (2002) Effects of simulated herbivory and resource availability on native and invasive exotic tree seedlings. Basic Appl Ecol 3:297–307
Rossiter-Rachur NA, Setterfield SA, Douglas MM, Hutley LB, Cook GD, Schmidt S (2009) Invasive Andropogon gayanus (gambagrass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecol Appl 19:1546–1560
Schmidt CD, Hickman KR, Channell R, Harmoney K, Stark W (2008) Competitive abilities of native grasses and non-native (Bothriochloa spp.) grasses. Plant Ecol 197:69–80
Scrifres CJ, Hamilton WT (1993) Prescribed burning for brushland management: the south Texas example. Texas A&M Univ Press, College Station
Siemann E, Rogers WE (2003) Changes in light and nitrogen availability under pioneer trees may indirectly facilitate tree invasions of grasslands. J Ecol 91:923–931
Simberloff D (1996) Impacts of introduced species in the United States. Consequences 2:13–22
Simmons MT, Windhager S, Power P, Lott J, Lyons RK, Schwope C (2007) Selective and non-selective control of invasive plants: the short-term effects of growing season, prescribed fire, herbicide, and mowing in two Texas prairies. Restor Ecol 15:662–669
Smeins FE, Diamond DD, Hanselka CW (1991) Coastal prairie. In: Coupland RT (ed) Ecosystems of the world 8A: natural grasslands. Elsevier, New York, pp 269–290
Soil and Plant Analysis Council (2000) Soil analysis handbook of reference methods. Soil and Plant Analysis Council, Inc., CRC Press, Boca Raton
Soil Survey Staff, Natural Resources Conservation Service (NRCS), United States Department of Agriculture (2010) Web soil survey of San Patricio and Aransas Counties, Texas. Available online at http://websoilsurvey.nrcs.usda.gov/. Accessed 10 Apr 2010
Stewart G, Hull AC (1949) Cheatgrass (Bromus tectorum L.)—an ecological intruder in southern Idaho. Ecology 30:58–74
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term impacts of species invasions. Trends Ecol Evol 21:645–651
Thébaud C, Finzi CA, Affre L, Debussche M, Escarre J (1996) Assessing why two introduced Conyza differ in their ability to invade Mediterranean old fields. Ecology 77:791–804
USDA, ARS (2011) National Genetic Resources Program. Germplasm Resources Information Network (GRIN [http://www.ars-grin.gov, 07 Sept 2011]). National Germplasm Resources Laboratory, Beltsville
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:468–478
Wedin DA, Tilman D (1990) Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84:433–441
White LM, Dewald CL (1996) Yield and quality of WW-Iron Master and causian bluestem regrowth. J Range Manag 49:42–45
Williams DG, Baruch Z (2000) African grass invasion in the Americas: ecosystem consequences and the role of ecophysiology. Biol Invasions 2:123–140
Zuo J, Rogers WE, DeWalt SJ, Siemann E (2006) Effects of Chinese tallow tree (Sapium sebiferum) ecotype on soil-plant system carbon and nitrogen processes. Oecologia 150:272–281
Acknowledgments
This research was supported by a graduate research fellowship from the Rob and Bessie Welder Wildlife Foundation, and this is Welder Wildlife Foundation contribution No. 706. We therefore, extend our gratitude to the Welder Wildlife Foundation and their staff for all their support. We thank Ryan Wilson for his assistance in the field, and Abe Levin-Nielson for his assistance with making the map for Fig. 1. This work also benefited from assistance and feedback provided by members of the McCulley grassland ecology lab and the ecosystem ecology discussion group at the University of Kentucky. Additional support for the project was provided by the University of Kentucky’s College of Agriculture, Departments of Plant and Soil Sciences and Forestry, and the Kentucky Agricultural Experiment Station (KY006045).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruffner, M.E., McCulley, R.L., Nelson, J.A. et al. Ecosystem function differs between Old World bluestem invaded and native coastal prairie in South Texas. Biol Invasions 14, 1483–1500 (2012). https://doi.org/10.1007/s10530-012-0174-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0174-4