Abstract
Nitrogenous derivatives of the two orange pigments from Monascus sp. with anti-melanogenic activities were prepared using fermentation and chemical synthesis. The pigments were produced in a 5 l jar fermentor. A total of 33 derivatives were synthesized via incorporation of L-amino acids and amines into the pigments. Two derivatives with high inhibitory melanin-synthesizing activities and low cell toxicities were selected based on testing using B16F10 cells. Glutamic acid and (S)-(+)-1-amino-2-propanol derivatives showed high inhibitory activities against melanogenesis. Both the reaction and expression of tyrosinase, an important enzyme in the melanin-synthesizing pathway, were inhibited by the glutamic acid derivative in a dose-dependent manner. The (S)-(+)-1-amino-2-propanol derivative inhibited expression of tyrosinase in cells, but not the tyrosinase reaction. TRP1 and TRP2, other important proteins in melanin-synthesis, were not affected by the two derivatives.






Similar content being viewed by others
References
Aytemir MD, Karakaya G (2012) Kojic acid derivatives. In: Ekinci D (ed) Medicinal chemistry and drug design. InTech, Croatia, pp 1–26
Chang T-S (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10:2440–2475
Chen L-G, Chang W-L, Lee C-J, Lee L-T, Shin C-M, Wang C-C (2009) Melanogenesis inhibition by gallotannins from Chinese galls in B16 mouse melanoma cells. Biol Pharm Bull 32:1447–1452
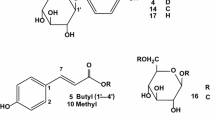
Choe D, Lee J, Woo S, Shin CS (2012) Evaluation of the amine derivatives of Monascus pigment with anti-obesity activities. Food Chem 134:315–323
Diawpanich P, Klongpityapong P, Veeranondha S, Watts P (2008) DMEM enhances tyrosinase activity in B16 mouse melanoma cells and human melanocytes. Songklanakarin J Sci Technol 30:603–609
Gillbro JM, Olsson MJ (2011) The melanogenesis and mechanism of skin-lightening agents—existing and new approaches. Int J Cosmet Sci 33:210–221
Hosi J, Abe E, Suda T, Kurok T (1985) Regulation of melanin synthesis of B16 mouse melanoma cells by 1α, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res 45:1474–1478
Huang H-C, Chiu S-H, Chang T-M (2011) Inhibitory effect of [6]-gingerol on melanogenesis in B16F10 melanoma cells and a possible mechanism of action. Biosci Biotechnol Biochem 75:1067–1072
Jeon J, Jung H, Kim JH, Kim YO, Youn SH, Shin CS (2008) Effect of the monascus pigment threonine derivative on regulation of the cholesterol level in mice. Food Chem 107:1078–1085
Jin YH, Lee SJ, Chung MH, Park JH, Park YI, Cho TH, Lee SK (1999) Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch Pharm Res 22:232–236
Jung H, Kim C, Kim K, Shin CS (2003) Color characteristics of monascus pigments derived by fermentation with various amino acids. J Agric Food Chem 51:1302–1306
Kim JH, Kim HJ, Jung H, Kim C, Kim YO, Ju JY, Shin CS (2007) Development of lipase inhibitors from various derivatives of monascus pigment produced by Monascus fermentation. Food Chem 101:357–364
Lee CL, Tsai TY, Wang JJ, Pan TM (2006) In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol 70:533–540
Moll HR, Farr DR (1976) Red pigment and process. US Pat 3,993,789
Satoko I, Nanaho H, Toshirou W, Naoki K, Akira Y, Hikoya H, Sakae AK (1997) Inhibitory effects of food-coloring agents derived from Monascus on the mutagenicity of heterocyclic amines. J Agric Food Chem 45:3980–3984
Staunton J, Weissman KJ (2001) Polyketide biosynthesis: a millennium review. Nat Prod Rep 18:380–416
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MOE) (No. 2013R1A1A2064267), the Ministry of Knowledge Economy (MKE), and the Korea Institute for Advancement of Technology (KIAT) through the Inter-ER Cooperation Projects.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jo, D., Choe, D., Nam, K. et al. Biological evaluation of novel derivatives of the orange pigments from Monascus sp. as inhibitors of melanogenesis. Biotechnol Lett 36, 1605–1613 (2014). https://doi.org/10.1007/s10529-014-1518-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1518-1