Abstract
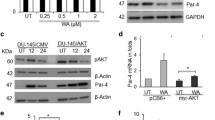
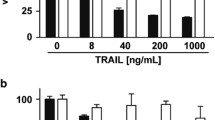
The overexpression of the pro-apoptotic protein Prostate Apoptosis Response Protein-4 in colon cancer has been shown to increase response to the chemotherapeutic agent 5-fluorouracil (5-FU). Although colon cancer cells endogenously express Par-4, the presence or overexpression of Par-4 alone does not cause apoptosis. We hypothesize that Par-4 is inactivated in colon cancer. In colon cancer, the levels and the kinase activity of the nonreceptor tyrosine kinase c-Src increase with tumor progression. One of the downstream effectors of c-Src is Akt1. Akt1 has been shown to inhibit the pro-apoptotic activity of Par-4 in prostate cancer cells. We therefore investigated the potential of activating Par-4 by inhibiting c-Src. Colon carcinoma cell lines were treated with the Src kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) in combination with the chemotherapeutic agent 5-FU. Treating cells with PP2 and 5-FU resulted in reduced interaction of Par-4 with Akt1 and with the scaffolding protein 14-3-3σ, and mobilization of Par-4 to the nucleus. Par-4 was shown to interact not only with Akt1 and 14-3-3σ, but also with c-Src. Overexpression of c-Src induced the phosphorylation of Par-4 at tyrosine site/s. Thus, in this study, we have shown that Par-4 can be activated by inhibiting Src with a pharmacological inhibitor and adding a chemotherapeutic agent. The activation of the pro-apoptotic protein Par-4 as reported in this study is a novel mechanism by which apoptosis occurs with a Src kinase inhibitor and 5-FU. In addition, we have demonstrated that the pro-apoptotic activity of endogenously expressed Par-4 can be increased in colon cancer cells.






Similar content being viewed by others
References
Pohl A, Zhang W, Ning Y, Manegold PC, Lurje G, Lenz HJ (2008) Targeting metastatic colorectal cancer in 2008: a long way from 5-FU. Oncology (Williston Park) 22(4):456–462 discussion 62–63, 67–68, 74 passim
Piedbois P, Buyse M (2008) Endpoints and surrogate endpoints in colorectal cancer: a review of recent developments. Curr Opin Oncol 20(4):466–471
Shrestha-Bhattarai T, Rangnekar VM (2010) Cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene 29(27):3873–3880
Sells S, Wood D, Joshi-Barve S, Muthukamar S, Jacob R, Crist S, Humphreys S (1994) Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ 5:457–466
Guo Q, Fu W, Xie J, Luo H, Sells S, Geddes J, Bondada V, Rangnekar V, Mattson M (1998) Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat Med 4:957–962
Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM (2005) Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell 20(1):33–44
Lee JW, Lee KF, Hsu HY, Hsu LP, Shih WL, Chu YC, Hsiao WT, Liu PF (2007) Protein expression and intracellular localization of prostate apoptosis response-4 (Par-4) are associated with apoptosis induction in nasopharyngeal carcinoma cell lines. Cancer Lett 257(2):252–262
Boehrer S, Chow K, Beske F, Kukoc-Zivojnov N, Puccetti E, Ruthardt M, Baum C, Rangnekar V, Hoelzer D, Mitrou P, Weidmann E (2002) In lymphatic cells Par-4 sensitizes to apoptosis by down-regulating Bcl-2 and promoting disruption of mitochondrial membrane potential and caspase activation. Cancer Res 62:1768–1775
Sells SF, Han SS, Muthukkumar S, Maddiwar N, Johnstone R, Boghaert E, Gillis D, Liu G, Nair P, Monnig S, Collini P, Mattson MP, Sukhatme VP, Zimmer SG, Wood DP Jr, McRoberts JW, Shi Y, Rangnekar VM (1997) Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol 17(7):3823–3832
Lee TJ, Lee JT, Kim SH, Choi YH, Song KS, Park JW, Kwon TK (2008) Overexpression of Par-4 enhances thapsigargin-induced apoptosis via down-regulation of XIAP and inactivation of Akt in human renal cancer cells. J Cell Biochem 103(2):358–368
Azmi AS, Philip PA, Zafar SF, Sarkar FH, Mohammad RM (2010) PAR-4 as a possible new target for pancreatic cancer therapy. Expert Opin Ther Targets 14(6):611–620
Azmi AS, Wang Z, Burikhanov R, Rangnekar VM, Wang G, Chen J, Wang S, Sarkar FH, Mohammad RM (2008) Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther 7(9):2884–2893
Franchitto A, Torrice A, Semeraro R, Napoli C, Nuzzo G, Giuliante F, Alpini G, Carpino G, Berloco PB, Izzo L, Bolognese A, Onori P, Renzi A, Cantafora A, Gaudio E, Alvaro D (2010) Prostate apoptosis response-4 is expressed in normal cholangiocytes, is down-regulated in human cholangiocarcinoma, and promotes apoptosis of neoplastic cholangiocytes when induced pharmacologically. Am J Pathol 177(4):1779–1790
Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, Linehan WM, Sukhatme VP, Weinstein MH, Rangnekar VM (1999) Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene 18(5):1205–1208
Fernandez-Marcos PJ, Abu-Baker S, Joshi J, Galvez A, Castilla EA, Canamero M, Collado M, Saez C, Moreno-Bueno G, Palacios J, Leitges M, Serrano M, Moscat J, Diaz-Meco MT (2009) Simultaneous inactivation of Par-4 and PTEN in vivo leads to synergistic NF-kappaB activation and invasive prostate carcinoma. Proc Natl Acad Sci USA 106(31):12962–12967
Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, Hardisson D, Diaz-Meco MT, Moscat J, Serrano M, Palacios J (2007) Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res 67(5):1927–1934
Saegusa M, Hashimura M, Kuwata T, Okayasu I (2010) Transcriptional regulation of pro-apoptotic Par-4 by NF-kappaB/p65 and its function in controlling cell kinetics during early events in endometrial tumourigenesis. J Pathol 221(1):26–36
Lee JW, Liu PF, Hsu LP, Chen PR, Chang CH, Shih WL (2009) EBV LMP-1 negatively regulates expression and pro-apoptotic activity of Par-4 in nasopharyngeal carcinoma cells. Cancer Lett 279(2):193–201
Gurumurthy S, Goswami A, Vasudevan KM, Rangnekar VM (2005) Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol Cell Biol 25(3):1146–1161
El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar V (2003) Identification of a unique core domain of Par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol 23:5516–5525
Kline CL, Jackson R, Engelman R, Pledger WJ, Yeatman TJ, Irby RB (2008) Src kinase induces tumor formation in the c-SRC C57BL/6 mouse. Int J Cancer 122(12):2665–2673
Talamonti MS, Roh MS, Curley SA, Gallick GE (1993) Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest 91(1):53–60
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE (2002) Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21(51):7797–7807
Golubovskaya VM, Gross S, Kaur AS, Wilson RI, Xu LH, Yang XH, Cance WG (2003) Simultaneous inhibition of focal adhesion kinase and SRC enhances detachment and apoptosis in colon cancer cell lines. Mol Cancer Res 1(10):755–764
Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M, Tilby MJ, Pearson DG, Ottley CJ, Workman P, Frame MC, Dive C (2004) Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem 279(44):46113–46121
Jiang T, Qiu Y (2003) Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J Biol Chem 278(18):15789–15793
Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y (2001) Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem 276(34):31858–31862
Chan TO, Tanaka A, Bjorge JD, Fujita DJ (1990) Association of type I phosphatidylinositol kinase activity with mutationally activated forms of human pp60c-src. Mol Cell Biol 10(6):3280–3283
Pleiman CM, Hertz WM, Cambier JC (1994) Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science 263(5153):1609–1612
Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB (2003) Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem 278(41):40057–40066
Kline CL, Shanmugavelandy SS, Kester M, Irby RB (2009) Delivery of PAR-4 plasmid in vivo via nanoliposomes sensitizes colon tumor cells subcutaneously implanted into nude mice to 5-FU. Cancer Biol Ther 8(19):1831–1837
Wang BD, Kline CL, Pastor DM, Olson TL, Frank B, Luu T, Sharma AK, Robertson G, Weirauch MT, Patierno SR, Stuart JM, Irby RB, Lee NH (2010) Prostate apoptosis response protein 4 sensitizes human colon cancer cells to chemotherapeutic 5-FU through mediation of an NF kappaB and microRNA network. Mol Cancer 9:98
Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ (1999) Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet 21(2):187–190
Leffers H, Madsen P, Rasmussen HH, Honore B, Andersen AH, Walbum E, Vandekerckhove J, Celis JE (1993) Molecular cloning and expression of the transformation sensitive epithelial marker stratifin. A member of a protein family that has been involved in the protein kinase C signalling pathway. J Mol Biol 231(4):982–998
van Hemert MJ, Niemantsverdriet M, Schmidt T, Backendorf C, Spaink HP (2004) Isoform-specific differences in rapid nucleocytoplasmic shuttling cause distinct subcellular distributions of 14-3-3 sigma and 14-3-3 zeta. J Cell Sci 117(Pt 8):1411–1420
Hermeking H (2003) The 14-3-3 cancer connection. Nat Rev Cancer 3(12):931–943
Oh JE, da Jang H, Kim H, Kang HK, Chung CP, Park WH, Min BM (2009) alpha3beta1 integrin promotes cell survival via multiple interactions between 14-3-3 isoforms and proapoptotic proteins. Exp Cell Res 315(18):3187–3200
Bridges D, Moorhead GB (2005) 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2005(296):re10
Basu S, Totty NF, Irwin MS, Sudol M, Downward J (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11(1):11–23
Libich DS, Schwalbe M, Kate S, Venugopal H, Claridge JK, Edwards PJ, Dutta K, Pascal SM (2009) Intrinsic disorder and coiled-coil formation in prostate apoptosis response factor 4. FEBS J 276(14):3710–3728
Obenauer JC, Cantley LC, Yaffe MB (2003) Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31(13):3635–3641
Campbell SJ, Jackson RM (2003) Diversity in the SH2 domain family phosphotyrosyl peptide binding site. Protein Eng 16(3):217–227
Puntervoll P, Linding R, Gemund C, Chabanis-Davidson S, Mattingsdal M, Cameron S, Martin DM, Ausiello G, Brannetti B, Costantini A, Ferre F, Maselli V, Via A, Cesareni G, Diella F, Superti-Furga G, Wyrwicz L, Ramu C, McGuigan C, Gudavalli R, Letunic I, Bork P, Rychlewski L, Kuster B, Helmer-Citterich M, Hunter WN, Aasland R, Gibson TJ (2003) ELM server: a new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res 31(13):3625–3630
Onofri F, Giovedi S, Vaccaro P, Czernik AJ, Valtorta F, De Camilli P, Greengard P, Benfenati F (1997) Synapsin I interacts with c-Src and stimulates its tyrosine kinase activity. Proc Natl Acad Sci USA 94(22):12168–12173
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11(7):36–42
Acknowledgments
This work was funded by a grant from the Jane B. Barsumian Trust (R. Irby) and a fellowship award from the American Foundation for Aging Research (C.L. Kline). The authors would also like to acknowledge Wade Edris and the Penn State Hershey College of Medicine Imaging Core for assistance in collecting the confocal microscope images in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kline, C.L.B., Irby, R.B. The pro-apoptotic protein Prostate Apoptosis Response Protein-4 (Par-4) can be activated in colon cancer cells by treatment with Src inhibitor and 5-FU. Apoptosis 16, 1285–1294 (2011). https://doi.org/10.1007/s10495-011-0648-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0648-3