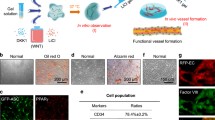
Abstract
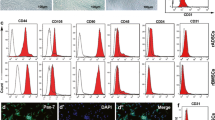
Using a fibrin-based angiogenesis model, we have established that there is no canonical mechanism used by endothelial cells (ECs) to degrade the surrounding extracellular matrix (ECM), but rather the set of proteases used is dependent on the mural cells providing the angiogenic cues. Mesenchymal stem cells (MSCs) originating from different tissues, which are thought to be phenotypically similar, promote angiogenesis through distinct mechanisms. Specifically, adipose-derived stem cells (ASCs) promote utilization of the plasminogen activator-plasmin axis by ECs as the primary means of vessel invasion and elongation in fibrin. Matrix metalloproteinases (MMPs) serve a purpose in regulating capillary diameter and possibly in stabilizing the nascent vessels. These proteolytic mechanisms are more akin to those involved in fibroblast-mediated angiogenesis than to those in bone marrow-derived stem cell (BMSC)-mediated angiogenesis. In addition, expression patterns of angiogenic factors such as urokinase plasminogen activator (uPA), hepatocyte growth factor (HGF), and tumor necrosis factor alpha (TNFα) were similar for ASC and fibroblast-mediated angiogenesis, and in direct contrast to BMSC-mediated angiogenesis. The present study illustrates that the nature of the heterotypic interactions between mural cells and endothelial cells depend on the identity of the mural cell used. Even MSCs which are shown to behave phenotypically similar do not stimulate angiogenesis via the same mechanisms.








Similar content being viewed by others
References
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257
Zandonella C (2003) Tissue engineering: the beat goes on. Nature 421(6926):884–886
Griffith CK et al (2005) Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng 11(1–2):257–266
Fuchs S et al (2001) Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol 37(6):1726–1732
Rehman J et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298
Ghajar CM, George SC, Putnam AJ (2008) Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr 18(3):251–278
Kroon ME et al (1999) Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol 154(6):1731–1742
Kroon ME et al (2000) Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: implications for capillary-like tube formation in a fibrin matrix. Blood 96(8):2775–2783
Kroon ME et al (2001) Vascular endothelial growth factor enhances the expression of urokinase receptor in human endothelial cells via protein kinase C activation. Thromb Haemost 85(2):296–302
Chun TH et al (2004) MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 167(4):757–767
Hotary KB et al (2002) Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med 195(3):295–308
Yancopoulos GD et al (2000) Vascular-specific growth factors and blood vessel formation. Nature 407(6801):242–248
Ghajar CM et al (2010) Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316(5):813–825
Crisan M et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313
Caplan AI (2008) All MSCs are pericytes? Cell Stem Cell 3(3):229–230
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217(2):318–324
Nakatsu MN, Hughes CC (2008) An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol 443:65–82
Ghajar CM et al (2006) Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng 12(10):2875–2888
Ghajar CM et al (2008) The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J 94(5):1930–1941
Zuk PA et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Galvez BG et al (2001) Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem 276(40):37491–37500
Grobelny D, Poncz L, Galardy RE (1992) Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31(31):7152–7154
Prentice CR (1980) Basis of antifibrinolytic therapy. J Clin Pathol Suppl (R Coll Pathol) 14:35–40
Davis GE, Saunders WB (2006) Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11(1):44–56
Stratman AN et al (2009) Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114(2):237–247
Yana I et al (2007) Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 120(Pt 9):1607–1614
Collen A et al (2003) Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood 101(5):1810–1817
Kniazeva E, Putnam AJ (2009) Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol 297(1):C179–C187
Niedbala MJ, Picarella MS (1992) Tumor necrosis factor induction of endothelial cell urokinase-type plasminogen activator mediated proteolysis of extracellular matrix and its antagonism by gamma-interferon. Blood 79(3):678–687
Kumar R et al (1998) Regulation of distinct steps of angiogenesis by different angiogenic molecules. Int J Oncol 12(4):749–757
Moriyama T et al (1999) Simultaneous up-regulation of urokinase-type plasminogen activator (uPA) and uPA receptor by hepatocyte growth factor/scatter factor in human glioma cells. Clin Exp Metastasis 17(10):873–879
Nishimura K et al (2003) Effects of hepatocyte growth factor on urokinase-type plasminogen activator (uPA) and uPA receptor in DU145 prostate cancer cells. Int J Androl 26(3):175–179
Lehti K et al (2005) An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev 19(8):979–991
Sounni NE et al (2010) Stromal regulation of vessel stability by MMP14 and TGFbeta. Dis Model Mech 3(5–6):317–332
Baluk P et al (2009) TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 119(10):2954–2964
Ruoslahti E (2002) Drug targeting to specific vascular sites. Drug Discov Today 7(22):1138–1143
Ruoslahti E (2004) Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans 32(Pt3):397–402
Phelps EA et al (2010) Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci USA 107(8):3323–3328
Moon JJ et al (2010) Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 31(14):3840–3847
Aust L et al (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6(1):7–14
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100(9):1249–1260
Acknowledgments
We are grateful to Dr. Alicia Garcia Arroyo for graciously providing us the MT1-MMP function-blocking antibody, Dr. Christopher Hughes and Linda Him for providing us with fresh umbilical cords, and Dr. Cyrus Ghajar and Ekaterina Kniazeva for providing insightful discussions. We would also like to thank Dr. Enrico Gratton and Dr. Michelle Digman for assistance with confocal reflectance imaging, along with Dr. Jan Stegemann and Dr. Michael Mayer for providing us access to their instrumentation. This work was supported by a grant from the National Institutes of Health (R01 HL085339).
Conflict of intrests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare that the experiments described within this manuscript comply with the current laws of the United States of America.
Rights and permissions
About this article
Cite this article
Kachgal, S., Putnam, A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14, 47–59 (2011). https://doi.org/10.1007/s10456-010-9194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-010-9194-9