Abstract
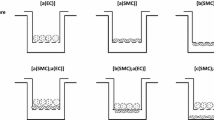
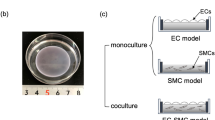
This study describes cocultures of arterial smooth muscle cells (SMCs) and endothelial cells (ECs) and the influences of their heterotypic interactions on hydraulic conductivity (L p ), an important transport property. A unique feature of these cocultures is that ECs were first grown to confluence and then SMCs were inoculated. Bovine aortic smooth muscle cells and bovine aortic endothelial cells (BAECs) were cocultured on Transwell Permeable Supports, and then exposed to a pressure-driven transmural flow. L p across each culture was measured using a bubble tracking apparatus that determined water flux (J v ). Our results indicate that arterial L p is significantly modulated by EC–SMC proximity, and serum content in culture. The L p of cocultures was also compared to the predictions of a resistances-in-series model to distinguish the contributions of heterotypic interactions between SMCs and ECs. Conditions that lead to significantly reduced coculture L p , compared to BAEC monoculture controls, have been uncovered and the lowest L p in the literature for an in vitro system are reported. In addition, VE-cadherin immunostaining of intact BAEC monolayers in each culture configuration reveals that EC–SMC proximity on a porous membrane has a dramatic influence on EC morphology patterns. The cocultures with the lowest L p have ECs with significantly elongated morphology. Confocal imaging indicates that there are no direct EC–SMC contacts in coculture.









Similar content being viewed by others
References
Aiello, V. D., P. S. Gutierrez, M. J. Chaves, A. A. Lopes, M. L. Higuchi, et al. Morphology of the internal elastic lamina in arteries from pulmonary hypertensive patients: a confocal laser microscopy study. Mod. Pathol. 16:411–416, 2003.
Alexander, J. S., W. F. Patton, B. W. Christman, L. L. Cuiper, and F. R. Haselton. Platelet-derived lysophosphatidic acid decreases endothelial permeability in vitro. Am. J. Physiol. 274:H115–H122, 1998.
Buschmann, I., and W. Schaper. Arteriogenesis versus angiogenesis: two mechanisms of vessel growth. News Physiol. Sci. 14:121–125, 1999.
Cancel, L. M., A. Fitting, and J. M. Tarbell. In vitro study of LDL transport under pressurized (convective) conditions. Am. J. Physiol. Heart Circ. Physiol. 293:H126–H132, 2007.
Castellot, Jr., J. J., M. J. Karnovsky, and B. M. Spiegelman. Potent stimulation of vascular endothelial cell growth by differentiated 3T3 adipocytes. Proc. Natl. Acad. Sci. U. S. A. 77:6007–6011, 1980.
Chang, Y. S., J. A. Yaccino, S. Lakshminarayanan, J. A. Frangos, and J. M. Tarbell. Shear-induced increase in hydraulic conductivity in endothelial cells is mediated by a nitric oxide-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 20:35–42, 2000.
Chiu, J. J., L. J. Chen, P. L. Lee, C. I. Lee, L. W. Lo, et al. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101:2667–2674, 2003.
Conway, E. M., D. Collen, and P. Carmeliet. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 49:507–521, 2001.
Conyers, G., L. Milks, M. Conklyn, and H. Showell. Cramer E.A factor in serum lowers resistance and opens tight junctions of MDCK cells. Am. J. Physiol. 259:C577–C585, 1990.
Davies, P. F., G. A. Truskey, H. B. Warren, S. E. O’Connor, and B. H. Eisenhaure. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J. Cell Biol. 101:871–879, 1985.
De Wit, C., M. Boettcher, and V. J. Schmidt. Signaling across myoendothelial gap junctions–fact or fiction? Cell Commun. Adhesion 15:231–245, 2008.
DeMaio, L., J. M. Tarbell, R. C. Scaduto, Jr., T. W. Gardner, and D. A. Antonetti. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 286:H731–H741, 2004.
Dora, K. A. Cell–cell communication in the vessel wall. Vasc. Med. 6:43–50, 2001.
Dull, R. O., H. Jo, H. Sill, T. M. Hollis, and J. M. Tarbell. The effect of varying albumin concentration and hydrostatic pressure on hydraulic conductivity and albumin permeability of cultured endothelial monolayers. Microvasc. Res. 41:390–407, 1991.
Duthu, G. S., and J. R. Smith. In vitro proliferation and lifespan of bovine aorta endothelial cells: effect of culture conditions and fibroblast growth factor. J. Cell. Physiol. 103:385–392, 1980.
Fagotto, F., and B. M. Gumbiner. Cell contact-dependent signaling. Dev. Biol. 180:445–454, 1996.
Fillinger, M. F., L. N. Sampson, J. L. Cronenwett, R. J. Powell, and R. J. Wagner. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J. Surg. Res. 67:169–178, 1997.
Gaballa, M. A., T. E. Raya, B. R. Simon, and S. Goldman. Arterial mechanics in spontaneously hypertensive rats. Mechanical properties, hydraulic conductivity, and two-phase (solid/fluid) finite element models. Circ. Res. 71:145–158, 1992.
Gartner, L. P., and J. L. Hiatt. Circulatory system. In: Color Textbook of Histology, edited by B. Schmitt. Philadelphia: Saunders Co., 2001, pp. 251–256.
Grazia Lampugnani, M., A. Zanetti, M. Corada, T. Takahashi, G. Balconi, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161:793–804, 2003.
Heberlein, K. R., A. C. Straub, and B. E. Isakson. The myoendothelial junction: breaking through the matrix? Microcirculation 16:307–322, 2009.
Heydarkhan-Hagvall, S., G. Helenius, B. R. Johansson, J. Y. Li, E. Mattsson, et al. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem. 89:1250–1259, 2003.
Hillsley, M. V., and J. M. Tarbell. Oscillatory shear alters endothelial hydraulic conductivity and nitric oxide levels. Biochem. Biophys. Res. Commun. 293:1466–1471, 2002.
Huang, Y., K. M. Jan, D. Rumschitzki, and S. Weinbaum. Structural changes in rat aortic intima due to transmural pressure. J. Biomech. Eng. 120:476–483, 1998.
Kurzen, H., S. Manns, G. Dandekar, T. Schmidt, S. Pratzel, et al. Tightening of endothelial cell contacts: a physiologic response to cocultures with smooth-muscle-like 10T1/2 cells. J. Investig. Dermatol. 119:143–153, 2002.
Langeler, E. G., and V. W. van Hinsbergh. Characterization of an in vitro model to study the permeability of human arterial endothelial cell monolayers. Thromb. Haemostasis 60:240–246, 1988.
Li, G., M. J. Simon, L. M. Cancel, Z. D. Shi, X. Ji, et al. Permeability of endothelial and astrocyte cocultures: in vitro blood–brain barrier models for drug delivery studies. Ann. Biomed. Eng. 38:2499–2511, 2010.
Mekata, F. Current spread in the smooth muscle of the rabbit aorta. J. Physiol. 242:143–155, 1974.
Minnear, F. L., S. Patil, D. Bell, J. P. Gainor, and C. A. Morton. Platelet lipid(s) bound to albumin increases endothelial electrical resistance: mimicked by LPA. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L1337–L1344, 2001.
Mortell, K. H., A. D. Marmorstein, and E. B. Cramer. Fetal bovine serum and other sera used in tissue culture increase epithelial permeability. In Vitro Cell. Dev. Biol. 29A:235–238, 1993.
Nitz, T., T. Eisenblätter, K. Psathaki, and H.-J. Galla. Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro. Brain Res. 981:30–40, 2003.
Pang, Z., and L. E. Niklason. Truskey. Porcine endothelial cells cocultured with smooth muscle cells became procoagulant in vitro. Tissue Eng. Part A 16:1835–1844, 2010.
Perez-Zoghbi, J. F., Y. Bai, and M. J. Sanderson. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J. Gen. Physiol. 135:247–259, 2010.
Powers, M. R., F. A. Blumenstock, J. A. Cooper, and A. B. Malik. Role of albumin arginyl sites in albumin-induced reduction of endothelial hydraulic conductivity. J. Cell. Physiol. 141:558–564, 1989.
Qazi, H., R. Palomino, Z. D. Shi, and L. L. Munn. Tarbell JM. Integr. Biol. (Camb): Cancer cell glycocalyx mediates mechanotransduction and flow-regulated invasion, 2013.
Renkin, E. M., and F. E. Curry. Endothelial permeability: pathways and modulations. Ann. N. Y. Acad. Sci. 401:248–259, 1982.
Rose, S. L., and J. E. Babensee. Complimentary endothelial cell/smooth muscle cell co-culture systems with alternate smooth muscle cell phenotypes. Ann. Biomed. Eng. 35:1382–1390, 2007.
Ryan, U. S., J. W. Ryan, and C. Whitaker. How do kinins affect vascular tone? Adv. Exp. Med. Biol. 120A:375–391, 1979.
Schaper, W., and I. Buschmann. Arteriogenesis, the good and bad of it. Cardiovasc. Res. 43:835–837, 1999.
Shields, J. D., M. E. Fleury, C. Yong, A. A. Tomei, G. J. Randolph, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11:526–538, 2007.
Tada, S., and J. M. Tarbell. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 278:H1589–H1597, 2000.
Tarbell, J. M. Shear stress and the endothelial transport barrier. Cardiovasc. Res. 87:320–330, 2010.
Tarbell, J. M., L. Demaio, and M. M. Zaw. Effect of pressure on hydraulic conductivity of endothelial monolayers: role of endothelial cleft shear stress. J. Appl. Physiol. 87:261–268, 1999.
Tarbell, J. M., M. J. Lever, and C. G. Caro. The effect of varying albumin concentration of the hydraulic conductivity of the rabbit common carotid artery. Microvasc. Res. 35:204–220, 1988.
van Oostrom, M. C., O. van Oostrom, P. H. Quax, M. C. Verhaar, and I. E. Hoefer. Insights into mechanisms behind arteriogenesis: what does the future hold? J. Leukoc. Biol. 84:1379–1391, 2008.
Wen, H., Y. Lu, H. Yao, and S. Buch. Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One 6:e21707, 2011.
Ziegler, T., R. W. Alexander, and R. M. Nerem. An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann. Biomed. Eng. 23:216–225, 1995.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant HL57093.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Peter E. McHugh oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Mathura, R.A., Russell-Puleri, S., Cancel, L.M. et al. Hydraulic Conductivity of Endothelial Cell-Initiated Arterial Cocultures. Ann Biomed Eng 42, 763–775 (2014). https://doi.org/10.1007/s10439-013-0943-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0943-y