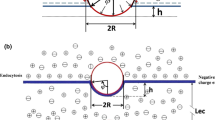
Abstract
Charge carried by the surface glycocalyx layer (SGL) of the cerebral endothelium has been shown to significantly modulate the permeability of the blood–brain barrier (BBB) to charged solutes in vivo. The cultured monolayer of bEnd3, an immortalized mouse cerebral endothelial cell line, is becoming a popular in vitro BBB model due to its easy growth and maintenance of many BBB characteristics over repeated passages. To test whether the SGL of bEnd3 monolayer carries similar charge as that in the intact BBB and quantify this charge, which can be characterized by the SGL thickness (L f ) and charge density (C mf ), we measured the solute permeability of bEnd3 monolayer to neutral solutes and to solutes with similar size but opposite charges: negatively charged α-lactalbumin (−11) and positively charged ribonuclease (+3). Combining the measured permeability data with a transport model across the cell monolayer, we predicted the L f and the C mf of bEnd3 monolayer, which is ~160 nm and ~25 mEq/L, respectively. We also investigated whether orosomucoid, a plasma glycoprotein modulating the charge of the intact BBB, alters the charge of bEnd3 monolayer. We found that 1 mg/mL orosomucoid would increase SGL charge density of bEnd3 monolayer to ~2-fold of its control value.








Similar content being viewed by others
References
Adamson, R. H., and G. Clough. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J. Physiol. 445:473–486, 1992.
Adamson, R. H., V. H. Huxley, and F. E. Curry. Single capillary permeability to proteins having similar size but different charge. Am. J. Physiol. 254(2 Pt 2):H304–H312, 1988.
Adamson, R. H., J. F. Lenz, and F. E. Curry. Quantitative laser scanning confocal microscopy on single capillaries: permeability measurement. Microcirculation 1(4):251–265, 1994.
Adamson, R. H., J. E. Lenz, X. Zhang, G. N. Adamson, S. Weinbaum, and F. E. Curry. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J. Physiol. Lond. 557(3):889–907, 2004.
Allt, G., and J. G. Lawrenson. Is the pial microvessel a good model for blood-brain barrier studies? Brain Res. Brain Res. Rev. 24(1):67–76, 1997.
Barakat, A. I. Dragging along: the glycocalyx and vascular endothelial cell mechanotransduction. Circ. Res. 102(7):747–748, 2008.
Brown, R. C., K. S. Mark, R. D. Egleton, J. D. Huber, A. R. Burroughs, and T. P. Davis. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFkappaB. J. Cell Sci. 116(Pt 4):693–700, 2003.
Brown, R. C., A. P. Morris, and R. G. O’Neil. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 1130(1):17–30, 2007.
Bruegger, D., M. Jacob, M. Rehm, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 289(5):H1993–H1999, 2005.
Butt, A. M. Effect of inflammatory agents on electrical resistance across the blood-brain barrier in pial microvessels of anaesthetized rats. Brain Res. 696(1–2):145–150, 1995.
Cabrales, P., B. Y. Vazquez, A. G. Tsai, and M. Intaglietta. Microvascular and capillary perfusion following glycocalyx degradation. J. Appl. Physiol. 102(6):2251–2259, 2007.
Cassella, J. P., J. G. Lawrenson, and J. A. Firth. Development of endothelial paracellular clefts and their tight junctions in the pial microvessels of the rat. J. Neurocytol. 26:547–575, 1997.
Curry, F. E., J. C. Rutledge, and J. F. Lenz. Modulation of microvessel wall charge by plasma glycoprotein orosomucoid. Am. J. Physiol. 257(5 Pt 2):H1354–H1359, 1989.
del Zoppo, G. J., and J. M. Hallenbeck. Advances in the vascular pathophysiology of ischemic stroke. Thromb. Res. 98(3):73–81, 2000.
Farkas, E., and P. G. Luiten. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 64(6):575–611, 2001.
Fu, B. M., R. H. Adamson, and F. E. Curry. Test of a two-pathway model for small-solute exchange across the capillary wall. Am. J. Physiol. 274(6 Pt 2):H2062–H2073, 1998.
Fu, B., and B. Chen. A model for the modulation of microvessel permeability by junction strands. J. Biomech. Eng. Trans. ASME 125(5):620–627, 2003.
Fu, B. M., B. Chen, and W. Chen. An electrodiffusion model for effects of surface glycocalyx layer on microvessel permeability. Am. J. Physiol. Heart Circ. Physiol. 284(4):H1240–H1250, 2003.
Fu, B. M., and S. Shen. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc. Res. 68(1):51–62, 2004.
Gil, E. S., J. Li, H. Xiao, and T. L. Lowe. Quaternary ammonium beta-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules 10:505–516, 2009.
Grabb, P. A., and M. R. Gilbert. Neoplastic and pharmacological influence on the permeability of an in vitro blood-brain barrier. J. Neurosurg. 82(6):1053–1058, 1995.
Hamann, G. F., Y. Okada, R. Fitridge, and G. J. del Zoppo. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke 26(11):2120–2126, 1995.
Haraldsson, B., and B. Rippe. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol. Scand. 129(1):127–135, 1987.
Hawkins, B. T., and T. P. Davis. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57(2):173–185, 2005.
Koto, T., K. Takubo, S. Ishida, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am. J. Pathol. 170(4):1389–1397, 2007.
Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 4(5):484–488, 2004.
Leblond, C. P., and S. Inoue. Structure, composition, and assembly of basement membrane. Am. J. Anat. 185(4):367–390, 1989.
Malina, K. C., I. Cooper, and V. I. Teichberg. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 1284:12–21, 2009.
Matsumoto, K., K. Nishi, M. Kikuchi, et al. Alpha1-acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol. Pharm. Bull. 30(7):1226–1230, 2007.
Miosge, N. The ultrastructural composition of basement membranes in vivo. Histol. Histopathol. 16(4):1239–1248, 2001.
Moghimi, S. M., A. C. Hunter, and J. C. Murray. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 53(2):283–318, 2001.
Nicolazzo, J. A., S. A. Charman, and W. N. Charman. Methods to assess drug permeability across the blood-brain barrier. J. Pharm. Pharmacol. 58(3):281–293, 2006.
Omidi, Y., L. Campbell, J. Barar, D. Connell, S. Akhtar, and M. Gumbleton. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 990(1–2):95–112, 2003.
Potter, D. R., and E. R. Damiano. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ. Res. 102(7):770–776, 2008.
Sann, L., F. Bienvenu, J. Bienvenu, J. Bourgeois, and M. Bethenod. Evolution of serum prealbumin, C-reactive protein, and orosomucoid in neonates with bacterial infection. J. Pediatr. 105(6):977–981, 1984.
Schnitzer, J. E., and E. Pinney. Quantitation of specific binding of orosomucoid to cultured microvascular endothelium: role in capillary permeability. Am. J. Physiol. 263(1 Pt 2):H48–H55, 1992.
Schulze, C., and J. A. Firth. Interendothelial junctions during blood-brain-barrier development in the rat—morphological-changes at the level of individual tight junctional contacts. Dev. Brain Res. 69(1):85–95, 1992.
Sorensson, J., G. L. Matejka, M. Ohlson, and B. Haraldsson. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am. J. Physiol. 276(2 Pt 2):H530–H534, 1999.
Squire, J. M., M. Chew, G. Nneji, C. Neal, J. Barry, and C. Michel. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 136(3):239–255, 2001.
Stevens, A. P., V. Hlady, and R. O. Dull. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am. J. Physiol. Lung Cell. Mol. Physiol. 293(2):L328–L335, 2007.
Tarbell, J. M., and M. Y. Pahakis. Mechanotransduction and the glycocalyx. J. Intern. Med. 259(4):339–350, 2006.
Thi, M. M., J. M. Tarbell, S. Weinbaum, and D. C. Spray. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc. Natl. Acad. Sci. USA 101(47):16483–16488, 2004.
Ueno, M., H. Sakamoto, Y. J. Liao, et al. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem. Cell Biol. 122(2):131–137, 2004.
Ueno, M., H. Sakamoto, H. Tomimoto, et al. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 107(6):532–538, 2004.
Weinbaum, S., X. Zhang, Y. Han, H. Vink, and S. C. Cowin. Mechanotransduction and flow across the endothelial glycocalyx. Proc. Natl. Acad. Sci. USA 100(13):7988–7995, 2003.
Yuan, W., G. Li, and B. Fu. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. FASEB J. 23:1020.1, 2009.
Yuan, W., Y. Lv, M. Zeng, and B. M. Fu. Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc. Res. 77(2):166–173, 2009.
Acknowledgments
This work is supported in part by the National Science Foundation CAREER award, the Andrew Grove Foundation and PSC-CUNY research award of the City University of New York. We also thank Dr. Tao Lowe and Dr. Eun Seok Gil for the QAβCD nanoparticles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Julia E. Babensee oversaw the review of this article.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10439-010-0134-z
Rights and permissions
About this article
Cite this article
Yuan, W., Li, G. & Fu, B.M. Effect of Surface Charge of Immortalized Mouse Cerebral Endothelial Cell Monolayer on Transport of Charged Solutes. Ann Biomed Eng 38, 1463–1472 (2010). https://doi.org/10.1007/s10439-010-9920-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9920-x