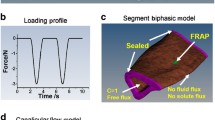
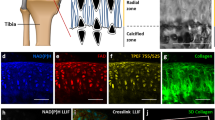
Abstract
Mechanical loading is well known to stimulate bone remodeling. Load-driven interstitial fluid flow and molecular transport have been postulated to play a role in the enhancement of bone formation. In order to evaluate load-driven molecular transport in a lacunocanalicular network, we conducted fluorescence recovery after photobleaching (FRAP) experiments using lacunae stained with uranine (376 Da). Loads were applied to a mouse femur ex vivo with a novel knee-loading modality, where the distal epiphysis was loaded with a sinusoidal force at 2 Hz. The lacunae in the diaphysis located 25% (∼4 mm) proximal to the loading site were photobleached and sequentially imaged, and a time constant for fluorescence recovery was determined both with and without knee loading. The time constant was estimated as the period to recover 63% of fluorescent intensity using a best-fit exponential curve. The results reveal that the applied loads shortened the time constant from 33 ± 9 s with non-loading control to 25 ± 11 s with knee loading (p = 0.0014). The strain in the measurement site was <100 μstain along the femoral midshaft, which was an order of magnitude smaller than the minimum effective strain threshold for bone remodeling. Taken together, the current study supports the notion that molecular transport in cortical bone is enhanced by the loads applied to the epiphysis without inducing significant in situ strain in the diaphysis.





Similar content being viewed by others
References
Adams G. R., Caiozzo V. J., Baldwin K. M., (2003) Skeletal muscle unweighting: Spaceflight and ground-based models J. Appl. Physiol. 95:2185–2201
Anderson E. J., Kaliyamoorthy S., Iwan J., Alexander D., Knothe Tate M. L., (2005) Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes Ann. Biomed. Eng. 33(1):52–62
Bassett C. A. L., Becker R. O., (1962) Generation of electric potentials in bone in response to mechanical stress Science 137:1063–1064
Becker R. O., (1972) Stimulation of partial limb regeneration in rats Nature 235:109–111
Burger E. H., Klein-Nulend J., (1999) Mechanotransduction in bone – role of the lacuno-canalicular network J. FASEB 13:S101–S112
Burr D. B., Robling A. G., Turner C. H., (2003) Effects of biomechanical stress on bones in animals Bone 30:781–786
Carmeliet G., Vico L., Bouillon R., (2001) Space flight: A challenge for normal bone homeostasis Crit. Rev. Eukaryot. Gene. Expr. 11:131–144
Gururaja S., Kim H. J., Swan C. C., Brand R. A., Lakes R. S., (2005) Modeling deformation-induced fluid flow in cortical bone’s canalicular–lacunar system Ann. Biomed. Eng. 33:7–25
Jendrucko R. J., Hyman W. A., Newell P. H., Chakrabarty B. K., (1976) Theoretical evidence for the generation of high pressure in bone cells J. Biomech. 9:87–91
Johnson M. W., Chakkalakal D. A., Harper R. A., Katz J. L., Rouhana S. W., (1982) Fluid flow in bone in vitro J. Biomech. 15:881–885
Kaspar D., Seidl W., Neidlinger-Wilke C., Claes L., (2000) In vitro effects of dynamic strain on the proliferative and metabolic activity of human osteoblasts J. Musculoskelet. Neuronal. Interact. 1:161–164
Knothe Tate M. L. (2001) Mixing mechanisms and net solute transport in bone Ann. Biomed. Eng. 29:810–811
Knothe Tate M. L., Steck R., Forwood M. R., Niederer P., (2000) In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation J. Exp. Biol. 203:2737–2745
Leddy H. A., Guilak F., (2003) Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching Ann. Biomed. Eng. 31:753–760
Mak A. F., Zhang J. D., (2001) Numerical simulation of streaming potentials due to deformation-induced hierarchical flows in cortical bone J. Biomech. Eng. 123:66–70
McCreadie B. R., Hollister S. J., Schaffler M. B., Goldstein S. A., (2004) Osteocyte lacuna size and shape in women with and without osteoporotic fracture J. Biomech. 37:563–572
Mishra S., Knothe Tate M. L., (2003) Effect of lacunocanalicular architecture on hydraulic conductance in bone tissue: Implications for bone health and evolution Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 273:752–762
Murphy N. M., Carroll P., (2003) The effect of physical activity and its interaction with nutrition on bone health Proc. Nutr. Soc. 62:829–838
Patel, R. B., J. M. O’Leary, S. J. Bhatt, A. Vasnja, and M. L. Knothe Tate. Determing the permeability of cortical bone at multiple length scales using fluorescence recovery after photobleaching techniques. 51st Ann. Meeting. ORS 141, 2004
Piekarski K., Munro M., (1977) Transport mechanism operating between blood supply and steocytes in long bones Nature 269:80–82
Robling A. G., Turner C. H., (2002) Mechanotransduction in bone: Genetic effects on mechanosensitivity in mice Bone 31:562–569
Steck R., Niederer P., Knothe Tate M. L., (2003) A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone J. Theor. Biol. 220:249–259
Su M., Yang L., Praveen R. S., Jiang H. H., Yokota H., (2005) Measurement of bone strain using electronic speckle pattern interferometry J. Holograph. Speckle 2:1–6
Tami A. E., Nasser P., Verborgt O., Schaffler M. B., Knothe Tate M. L., (2002) The role of interstitial fluid flow in the remodeling response to fatigue loading J. Bone. Miner. Res. 17:2030–2037
Tanaka S. M., Sun H. B., Yokota H., (2004) Bone formation induced by a novel form of mechanical loading on joint tissue Biol. Sci. Space 18:41–44
Turner C. H., (1998) Three rules for bone adaptation to mechanical stimuli Bone 23:399–407
Wang L., Cowin S. C., Weinbaum S., Fritton S. P., (2000) Modeling tracer transport in an osteon under cyclic loading Ann. Biomed. Eng. 28:1200–1209
Wang L., Wang Y., Han Y., Henderson S. C., Majeska R. J., Weinbaum S., Schaffler M. B., (2005) In situ measurement of solute transport in the bone lacunar–canalicular system Proc. Natl. Acad. Sci. USA 102(33):11911–11916
Yokota H., Tanaka S. M., (2005) Osteogenic potentials with joint loading modality J. Bone. Miner. Metab. 23:302–308
You L., Cowin S. C., Schaffler M. B., Weinbaum S., (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix J. Biomech. 34:1375–1386
You J., Yellowley C. E., Donahue H. J., Zhang Y., Chen Q., Jacobs C. R., (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow J. Biomech. Eng. 122:387–393
Acknowledgments
Imaging and FRAP experiments were conducted at The Indiana Center for Biological Microscopy. This study was in part supported by AR052144 and Indiana 21st Century Research and Technology Funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, M., Jiang, H., Zhang, P. et al. Knee-Loading Modality Drives Molecular Transport in Mouse Femur. Ann Biomed Eng 34, 1600–1606 (2006). https://doi.org/10.1007/s10439-006-9171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9171-z