Abstract
Centrifugal microfluidics has emerged as a unique approach to the development of integrated total analysis systems for medical diagnostics. However, despite its many advantages, the platform has a size limitation due to the centripetal pumping mechanism in which fluids can only be moved from the center of the disc to the rim. This limits the footprint of the microfluidic network to one radius of the disc, and this in turn limits the amount of space available to embed complex assays. In order to overcome this space limitation problem, we are developing new techniques to pump fluids back toward the center of the disc as to allow greater path lengths for the fluidic network. This study presents a novel pumping mechanism for centrifugal microfluidics utilizing a combination of centrifugation and pneumatic compression. Pneumatic energy is stored during high-speed centrifugation with sample fluids trapping then compressing air in specially designed chambers. The accumulated pneumatic energy is released by spinning down, which expands the trapped air and thus pumps liquids back toward the center of the CD. This newly developed method overcomes current limitations of centripetal pumping avoiding external manipulation or surface treatments. In this article, we explore the design of appropriate chambers to induce pneumatic pumping and analytically describe the mechanics behind the pumping action. For proof of principle, we have applied pneumatic pumping to siphon priming.
Similar content being viewed by others
1 Introduction
For over 40 years, the centrifugal microfluidic platform, otherwise known as compact disc (CD) fluidics, has been a research topic in both academia and industry. Today, CD microfluidics is emerging as an advanced system for lab-on-a-chip systems primarily geared toward biomedical applications, such as clinical biochemistry in blood diagnostics (Abaxis 2009) and rapid immunoassays (Gyros AB 2009). In “lab-on-a-CD” systems, complex assays are embedded in microfluidic networks on a plastic disc presenting many advantages over alternative approaches for miniaturization of sample-to-answer systems also known as micro total analysis systems (μTAS). For example, in the case of in vitro diagnostics, multiplexing of diverse and complex analytical functions is easily achieved by embedding several individual assays and running them simultaneously on identical segments symmetrically arranged on the same CD. Additionally, native CD optics can easily be adapted and modified to interface with disc-based assays enabling the automation of multiple analysis steps from sample preparation to detection (Bañuls et al. 2008; Morais et al. 2007).
The CD platform relies on centrifugal forces propelling fluids in fluidic manifolds from the center of the disc to the rim with the use of any external pumps. Centripetal pumping uses simple inexpensive motors that are programmed for hands free control and are readily available from the mature compact disc industry. Compared to the more common electrokinetic pumping platforms, centripetal pumping does not require high-voltage power supplies, and the propulsion force is not dependent on such difficult to control parameters as the pH or ionic strength of the sample and the surface charges of the fluid conduit. In order to illustrate the latter point, a wide range of fluids have indeed been successfully pumped using the microfluidic CD platform; this includes aqueous solutions, solvents, surfactants, and biological fluids (e.g., blood, mucus, urine, milk) (Madou et al. 2006). In general, a very attractive attribute of the centrifugal platform is the effective way in which it allows for the parallel manipulation of small amounts of liquids (Madou et al. 2006; Zoval and Madou 2004).
Although technologies supporting CD fluidics have expanded fast, especially in the last 10 years, important limitations do remain, not the least of which pertains to the one-directional nature of centripetal flow. In centripetal flow, fluids only move from the center to the edge of the disc, limiting the number of analytical steps that are possible. Prime examples include diagnostic assays with large sample volumes and those tests that involve many processing steps. In order to solve this problem, one must either miniaturize further, which will necessitate higher spin rates and more accurate machining, or find ways to bring liquids back to the center of the disc. By driving fluids back toward the CD center and then driving them outwards again through centrifugal pumping, fluids can make multiple passes utilizing the available CD space more efficiently.
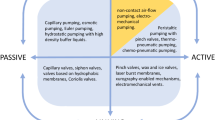
We considered both active (i.e., requires an additional actuator) and passive means (i.e., without an additional actuator) for fluid transfer back toward the center of the CD. The literature is bare of active pump designs except for a centrifugal-magnetic pump developed by Haeberle et al. (2007). The Haeberle system relies on permanent magnets embedded in a flexible plastic membrane fixed onto the CD surface. The permanent magnets in the flexible polymer sheet oscillate in a magnetic field modulated by the rotation of the CD above a set of fixed magnets in a stationary platform below the CD. The displacement of the magnets opens and closes valves that introduce air into the fluidic network to help push fluids through microchannels in the CD. Specifically, this design was developed for breaking up sample fluids by adding air bubbles (Haeberle et al. 2007). It is possible that this pumping method could be used as a mechanism for driving fluids back toward the CD center. However, this method requires that magnets be incorporated onto and below the CD, and that an additional material be added to the CD (a flexible polymer such as polydimethylsiloxane, PDMS, is often used for this purpose). Moreover, the introduction of air for pumping may initiate reactions with the sample fluids; this is of particular concern for diagnostics that are easily contaminated by ambient air.
Capillary action constitutes one way to achieve passive pumping of fluids back to the center of the CD. If the surface microchannel has a high enough surface energy, fluid flow can be induced through capillary forces (Ducrée et al. 2007), and, when the capillary forces are higher than the centrifugal forces, fluids can actually flow back toward the center of the CD. In order to create a high enough surface energy, the microchannels need to be made hydrophilic. Typically, the materials used in CD fabrication are hydrophobic [e.g., polycarbonate (PC) and polydimethylsiloxane (PDMS)] necessitating a surface treatment to make them hydrophilic. Several techniques are available to render a hydrophobic surface hydrophilic, including the deposition of a hydrophilic coating on the hydrophobic surface and the modification of the hydrophobic surface through chemical or physical surface treatments (Ducrée et al. 2007; Kido et al. 2007). A severe limitation comes about in the latter case, because surface treatments such as the exposure of polycarbonate to oxygen (O2) plasma are not permanent and result in a change of the fluidic behavior of the system over time (Larsson and Dérand 2002; Beaulieu et al. 2009). Typical treatments do degrade in a matter of days, thereby hindering the intended functionality of the CD (Larsson and Dérand 2002).
In order to create the next generation of microfluidic CD platforms that are capable of easily and efficiently pumping fluids back toward the center, new pumping systems have to be devised. Here, we report on a novel pumping mechanism that takes advantage of pneumatic energy storage and release to pump fluids back toward the center of a CD. This new technique, which we call pneumatic pumping, eliminates the need for an additional actuator and importantly it also obviates the need for any special added materials or surface treatments. In this report, we also present a theoretical analysis of pneumatic pumping and illustrate its utility for the priming of siphon valves.
2 Experimentation
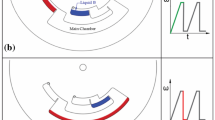
The basis of our newly introduced pumping mode is a specially designed fluidic manifold with two sub-compartments, i.e., an intake and a compression sub-compartment as illustrated in Fig. 1. An explanation of how the system works is presented in the schematic in Fig. 2. Fluids are initially introduced into the loading chamber (Fig. 2a). The disc is then spun to transfer the liquid to the pneumatic pumping chamber. The intake sub-compartment allows fluids to enter the system during centrifugal pumping (Fig. 2b). During continued filling, the fluid traps air in the compression sub-compartment (Fig. 2c). At high spin speeds, the trapped air is compressed and pressure builds up in the compression sub-compartment (Fig. 2d). By returning to a lower spin speed, the pressure on the liquid plug is relieved, causing the air in the compression chamber to expand, which in turn allows sample fluids to be pumped back toward the center of the CD (Fig. 2e).
Schematic of pneumatic pumping. a Loading of sample fluids. b Spinning the CD causes filling through the intake sub-compartment. c Continued filling eventually traps air the compression sub-compartment. d High speed centrifugation causes compression of the air in the compression sub-compartment, which allows near equalization of the fluid levels. e Slowing the CD down reduces the pressure on the compressed air, allowing expansion of the air. Air expansion causes pumping of the fluids back toward the CD center. Note in order to present a clearer illustration to the reader the approximate liquid levels are shown, and the venting hole from Fig. 1 is omitted
Experiments were performed to analyze how trapped air in the compression sub-compartment compressed over a range of rotational speeds. The microfluidic CDs were fabricated from a layered assembly of plastic discs featuring reservoir chambers and adhesive films incorporating the microchannels (Fig. 3). Using a QuickCircuit 5000 CNC router machine (T-Tech Corporation, USA), discs with chambers were machined in standard polycarbonate sheets (McMaster-Carr, USA). A vinyl cutter (Graphtec CE-2000, Graphtec America Inc., USA) was then employed to generate microchannel patterns in the pressure sensitive adhesive (DFM 200 clear 150 POLY H-9V-95, FLEXcon, USA). Thus the obtained parts were aligned and pressed together, and the adhesive layers were bonded the plastic discs together. The final five-layer CD system consists of three polycarbonate discs and two pressure sensitive adhesive layers: a top plastic disc (thickness 1.1 mm) contains the inlet, outlet, and venting holes; an adhesive layer (thickness 100 μm) contains the microchannels; the middle plastic disc (thickness 1.1 mm) holds the sample reservoirs; and a bottom adhesive (thickness 100 μm) and plastic disc (thickness 1.1 mm) seal off the system.
The experimental setup consists of a spin-stand used to spin the CDs and a visualization setup to observe fluidic movement. The spin-stand is equipped with a servo motor (Pacific Scientific, Model PCM21B) and an amplifier/controller (PAC SCI Programmable Servo Drive) to allow for various programmed spin profiles through the use of the ToolPAC (Pacific Scientific) interface software. For visualization, a digital video recording system is implemented, consisting of a high speed camera (Basler A301bc, 640 × 480 pixels, 80 fps max, 10× zoom lens mounted), a strobe light (PerkinElmer MVS-4200, 6 μs duration), and a retro-reflective fiber-optic sensor (Banner D10 Expert Fiber-Optic Sensor). In order to capture images, a reflective mark placed on the CD edge was used to actuate the fiber-optic sensor. During rotation, the reflective mark passes underneath the sensor triggering the camera and strobe light to capture one image frame per revolution. The images of the fluid flow are captured by the camera and subsequently transferred to a computer for data storage.
The pneumatic pumping experiments were carried out with 65 μl of colored dye (Neon food dye, McCormick, US) loaded onto the CD. The dye-loaded CD is placed on the servo motor on top of a separate reference disc, and both discs are mounted on the same custom aluminum spin chuck. Concentric rings separated at 1 mm distances are milled in the reference disc, to provide a visual reference for fluid positions during high speed centrifugation. In order to start an experiment, we first transfer the loaded dye into the compression sub-compartment by spinning the CD at the maximum speed of 7,000 rpm. Subsequently, the CD was spun at a range of spin speeds from 0 to 7,000 rpm, while the positions of the liquid levels at each steady spin speed in the loading sub-compartment were visually recorded. After image acquisition, movie frames were extracted showing the position of the liquid in the intake chamber at 500 rpm intervals (A series of still images showing the system conditions at 1,000 rpm intervals is shown in Fig. 4a–h). The measurements, run in triplicate, were used to verify our analytical analysis.
a–h Series of still images showing the liquid levels in the intake and compression sub-compartments of the pneumatic pumping device. The concentric rings observed are on a separate reference disc placed below the experimental CD. As the spin speeds increase, more pressure is induced on the liquid plug causing more air to be compressed. The limit of 7,000 rpm was due to the recommended maximum spin conditions for the motor
3 Analytical analysis
Here, we present a theoretical analysis of pneumatic pumping to provide guidance for the implementation of such systems for CD fluidics. The critical component in the pneumatic pumping design is the trapped air volume in the compression sub-compartment. Knowing the volume of that trapped air allows one to examine the pressures required for compression of the trapped air at different spin speeds. The pressure difference between the pressure in the initial condition (before compression) and that at a given spin speed is given by the Boyle equation. For the isothermal thermodynamic process illustrated in Fig. 5, this is given as:
where p 0 is the pressure of the trapped air in the CD is at rest (before compression), while p is the pressure of the air while spinning. Similarly, V 0 is the volume of the air in the compression sub-compartment when the CD is at rest. The volume V of air in the sub-compartment when the CD is spinning (compressing the air) in Eq. 1 is defined as:
In Fig. 5, we define \( x = R_{1} - R_{01} \) and \( y = R_{02} - R_{2} , \) i.e., the respective displacements of the liquid at a certain rotation frequency at the intake and compression sub-compartments. If we denote the cross-sectional area of the intake with S 1 and the compression sub-compartment with S 2, it becomes obvious that for an incompressible liquid:
The hydrostatic equilibrium at the level \( R_{02} - y \) (that is the condition where the hydrostatic pressure has the same value on the same CD circumference) is given as:
For convenience, we denote
where \( y = x{\frac{{S_{1} }}{{S_{2} }}}. \) We substitute Eq. 4 in Eq. 1 and take into account Eq. 3 to obtain:
Dividing Eq. 6 by p 0 V 0 gives
In general, this is a cubic equation of x which can now be solved; however, in the design presented in Fig. 1, the initial volume of the compression sub-compartment, V 0, requires clearer identification.Footnote 1 By redefining the semicircular area at the top of the compression sub-compartment in terms of a rectangle of equivalent area as shown in Fig. 6, V 0 is expressed as:
where R 0 is the position of the equivalent rectangular compression sub-compartment. A simple proof is given. The area of a semicircle and rectangle is equal \( {\frac{\pi }{2}}\left( {{\frac{w}{2}}} \right)^{2} = wh, \) where w represents the width of the rectangle (and the width of the compression sub-compartment) and h represents the rectangle’s height. R 0 can be found adding R c, defined as the position at the top of the semicircle to the difference of the radius of the semicircle, and h:
For simplicity, R 0 is left as a variable. Substituting Eq. 8 into Eq. 7 gives:
This expression can be solved numerically using the bisection method (Press et al. 2007); however, boundary conditions must be set to find the solution of x that falls in a real physical interval. In order to find the maximum x value, we reexamine Eq. 3 during the highest spin condition, where maximum air compression is occurring:
As a result of the compression, the liquid levels in both sub-compartments are approximately equal which additionally gives the following condition:
Solving the two equations for x max gives:
If k is defined as \( {\frac{{S_{1} }}{{S_{2} }}} \) then the interval of interest corresponds to \( \left[ {0,{\frac{{R_{02} - R_{01} }}{k + 1}}} \right]. \)
In our analysis, we used the following values: the density of the liquid ρ was taken for water, i.e., 1000 kg/m3; for the pressure of the relaxed air, p 0, we used standard air pressure, i.e., 101,300 N/m2; for the chamber dimensions, the intake sub-compartment area S 1 equals 1.216 mm2 and the area of the compression sub-compartment S 2 equals 6.08 mm2. Additionally, the width of the compression chamber w is 5 mm. R c equals 25.5 mm while R 02 equals 26 mm. R 01, which is highly dependent on the initial volume, was visually determined to be around 28.4 mm. Next, we will contrast the analytical values thus obtained with our experiments.
4 Results and discussion
The visual measurements of liquid plug positions in the intake sub-compartment approximated the predicted results from the analytical analysis presented above. This may be gleaned from the graph in Fig. 7 showing the positions of the liquid plug versus rotational speeds. For further clarification, the right axis of the graph additionally shows the volume of compressed air at different spin speeds.
Graph representing the comparison of experimental and theoretical values. The left axis of the graph corresponds to the liquid position of the intake fluid level versus the rotational frequency. There was good agreement between the experimental (represented by dots with error bars) and analytical values (plotted solid line). The right axis corresponds to the volume of air in the compression chamber versus rotational frequency
This analytical treatise provides a guide for designing the pneumatic pumping chamber. By evaluating different chamber geometries with known liquid sample volumes, the initial required compressed air volume can be determined. Furthermore, by comparing the fluid movement in particular stages of compression, the maximum and minimum liquid level positions in the intake sub-compartment are attained. These extremes define the disc real estate that must be implemented for pneumatic pumping in a particular design.
During attempts to implement the optimized designs for pneumatic pumping, two problems were identified and solved. First, depending on the exact filling pattern of the liquid manifold, surplus air may be trapped in the compression sub-compartment of the disc which makes accurate analysis impossible. More specifically, without a vent, air may be trapped in the channel connecting the intake and compression sub-compartments, instead of the compression sub-compartment alone. Adding venting holes to the intake sub-compartment eliminated this irreproducible loading issue. A second problem is encountered when changing the initial rotation direction. If the original spin direction is changed from counterclockwise to clockwise, after fluid is loaded into the pumping chamber, liquids were shifted to the compression sub-compartment from the intake sub-compartment. This shift is most likely due to a combination of Euler and Coriolis forces during the transition from higher steady spin speeds through deceleration to lower steady spin speeds. The movement of the liquids displaced some of the trapped air from the compression sub-compartment creating air bubbles which escape to the intake sub-compartment and to the air vents. During repeated cycling, the loss of trapped air reduces the amount of stored energy created during centrifugation, dropping the efficiency of the system. In order to avoid this loss of trapped air escape, the CD was only rotated in a unidirectional counterclockwise fashion thus eliminating air losses. Therefore, an important consequence of this phenomenon is that the position of the compression sub-compartment with respect to the intake sub-compartment dictates the rotation direction. For example, if the compression sub-compartment is designed to be on the right side of the intake sub-compartment, then the CD must be spun in the counterclockwise direction for optimal performance. It should be noted that both issues presented deal with the dynamics of air/liquid interfaces in closed chambers embedded in centrifugal microfluidics. As highlighted in recent advances, this topic remains relevant in the field and warrants more complete investigation (Mark et al. 2009).
The pneumatic pumping technique provides a novel method for pumping fluids back to the center of the CD without the need for external pumping manipulation or the use of surface treatments. Current centrifugal platforms, especially integrated CD-based in vitro diagnostic (IVD) devices, use surface treatments to implement siphon valves (Abaxis 2009; Ducrée et al. 2007; Kido et al. 2007; Steigert et al. 2007). These siphon microchannels are typically plasma treated and the hydrophilicity thus induced changes over time. Pneumatic pumping obviates the need for surface treatments.
Other applications of pneumatic pumping include its use in rapid mixing of fluids in microsystems (Kellogg and Carvalho 2003; Noroozi et al. 2009). In Noorozi et al., the authors showed efficient mixing by oscillating fluids through a mixing chamber by repeated pneumatic pumping and centrifugation (Noroozi et al. 2009). Their system addresses a key issue in microfluidics as micromixing becomes difficult in the microdomain due to low Reynolds numbers and hence predominately laminar flow. Even though the latter example constitutes a good potential application, it does not demonstrate the possibility for further downstream processing yet. It is in this context that we made an attempt to integrate pneumatic pumping with further processing of the pumped sample as in siphon priming.
5 Application—siphon priming
In CD microfluidics, siphoning is often used to pump fluids; for example, to define sample volumes (Ducrée et al. 2007; Kido et al. 2007). Additionally, siphon designs in centrifugal microfluidics provide a valving solution for CD applications that require high spin speeds during initial steps of the operation (e.g., plasma separation from whole blood) (Abaxis 2009; Steigert et al. 2007). Such high spin speed in early assay steps prevents the use of other valves operating at lower speeds.
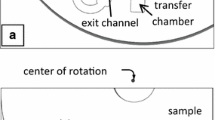
In a CD, siphoning works by allowing liquids to drain from a loading reservoir to a collection reservoir through a microchannel that has a crest at a point closer to the CD center than the position of the liquid level in the loading reservoir as illustrated in the siphon schematic in Fig. 8. Notice a capillary valve (i.e., a widening of the flow conduit) at the exit of the siphon microchannel before it empties out in the collection reservoir providing for better fluid control. When the microchannel is primed, in other words, it is filled with liquid, and the microchannel drain is further from the CD center than the position of the liquid level in the loading reservoir, then fluid will transfer under rotation to the collection reservoir. The condition sine qua non for liquid transfer to occur is that the siphon primes. The most common ways to prime a siphon (i.e., force fluids over the siphon crest) are to hydrostatically force the liquid to overflow the siphon crest or to use capillary priming (often with treated hydrophilic surfaces). In the latter case, low spin speed or keeping the disc at rest (Ducrée et al. 2007) is required, because the centrifugal force opposes the capillary force preventing priming at high spin speeds. In our design, we use the pneumatic energy release as a way to prime the siphon, eliminating the need for hydrostatic priming or surface treatments.
Schematic showing a siphon structure on a disc. During high-speed rotation, fluids are held in the bottom part of the loading reservoir and in the entrance to the siphon microchannel. Once the sample fluid reaches the end of the microchannel, the siphon is primed. At this point, the liquid is restrained by the capillary valve. Increasing the speed of rotation breaks the capillary valve and empties the fluid from the loading to the collection reservoir
In a pneumatic pump design, fluid is pumped toward the CD center as a result of the release of stored energy created during rotation as illustrated in Fig. 9. First, the loading chamber is filled with sample solution (Fig. 9a). Next, spinning the CD propels sample fluids toward the CD rim causing the intake sub-compartment to fill (Fig. 9b). As the fluid continues to flow, the sample liquid traps air in a compression sub-compartment (Fig. 9c). In case the CD rotation speeds are kept low, the fluid loads the pneumatic chamber without any compression of the trapped air and immediately primes the siphon. However, if rotational speeds are high enough, the centrifugal force exerted on the fluid pressurizes the trapped air. At a critical rotation frequency, the fluid levels in the two sub-compartments nearly equalize and therefore at that point the maximum air compression is achieved (Fig. 9d). If the rotational frequency is reduced now, the centrifugal force on the sample fluids becomes lower and fluid is pumped back toward the center of the disc due to the relaxation of the pressured air in the compression reservoir. By continued spinning at low speeds, the fluid overcomes the siphon crest and primes the siphon (Fig. 9e). Slightly increasing the rotational frequency breaks the capillary valve at the end of the siphon capillary and causes fluid to enter into the collection chamber. Continued centrifugation at a yet higher frequency completely empties the pneumatic pumping chamber (Fig. 9f).
Schematics and time-lapse images of the pneumatic pumping design coupled with a siphon valve. a Sample loading. b Filling of pneumatic pumping chamber under high rotational speeds (7,000 rpm). c Trapping of air in the compression sub-compartment of the chamber. d Compression of air in the compression sub-compartment with equalization of liquid levels. e Lowering the spin speeds (~1,000 rpm) causes relaxation of air in compression sub-compartment and pumping of liquid levels in intake sub-compartment toward the center of the disc. Simultaneous priming of the siphon takes place when liquid levels rise above the crest of the siphon. f Continued spinning empties pneumatic pumping chamber. A complete video of pneumatic pumping with siphoning can be seen in Movie A
In our experiments, we found pneumatic pumping to be an efficient way of siphon priming in a centrifugal device. By taking into account the earlier introduced analysis of the compression conditions, a siphon valve is easily implemented. Design parameters for siphon valves can be found in recent work that details siphon theory on centrifugal platforms (Siegrist et al. 2009).
6 Conclusions
The work presented here constitutes a novel way of creating pumping forces on a CD that can propel fluids back toward the disc center against the centrifugal forces that dominate in CD microfluidics. While other pumping techniques exist to return fluids to the center of the disc, they rely on surface modifications of the fluidic channels or on additional actuators integrated on the CD. Our technique works purely by trapping pneumatic energy created during CD rotation and released to pump fluids.
The pneumatic pumping method was modeled, and the model was confirmed by analyzing pumping positions and air compression versus spin speeds. The use of a pneumatic pumping mechanism for priming siphons was used as a practical application of this new pumping means. Our investigations prove that the manipulation of pneumatic energy is an efficient way for pumping fluids on “Lab-on-a-CD” platforms. Current platforms are limited in experimental size due to the lack of pumping techniques that pump fluids back to the center of the disc, and our design enables that allowing for more complex CD-based technologies.
Notes
Although a rectangular shape would be optimal for easy analysis, CNC milling creates rounded edges which cannot be avoided.
References
Abaxis Inc., USA (2009) Available: www.abaxis.com
Bañuls M, García-Piñón F, Puchades R, Maquieira Á (2008) Chemical derivatization of compact disc polycarbonate surfaces for SNPs detection. Bioconjug Chem 19:665–672
Beaulieu I, Geissler M, Mauzeroll J (2009) Oxygen plasma treatment of polystyrene and zeonor: substrates for adhesion of patterned cells. Langmuir 25:7169–7176
Ducrée J, Haeberle S, Lutz S, Pausch S, Stetten Fv, Zengerle R (2007) The centrifugal microfluidic bio-disk platform. J Micromech Microeng 17:S103–S115
Gyros AB, Sweden (2009) Available: www.gyros.com
Haeberle S, Schmitt N, Zengerle R, Ducrée J (2007) Centrifugo-magnetic pump for gas-to-liquid sampling. Sens Actuators A 135:28–33
Kellogg G, Carvalho B (2003) Bidirectional flow centrifugal microfluidic devices. US Patent No. 6527432
Kido H, Micic M, Smith D, Zoval J, Norton J, Madou M (2007) A novel, compact disk-like centrifugal microfluidics system for cell lysis and sample homogenization. Colloids Surf B 58:44–51
Larsson A, Dérand H (2002) Stability of polycarbonate and polystyrene surfaces after hydrophilization with high intensity oxygen RF plasma. J Colloid Interface Sci 246:214–221
Madou M, Zoval J, Jia G, Kido H, Kim J, Kim N (2006) Lab on a CD. Annu Rev Biomed Eng 8:601–628
Mark D, Tobias M, Haeberle S, Lutz S, Ducrée J, Zengerle R, von Stetten F (2009) Centrifugo-pneumatic valve for metering of highly wetting liquids on centrifugal microfluidic platforms. Lab Chip 9:3599–3603
Morais S, Carrascosa J, Mira D, Puchades R, Maquieira A (2007) Microimmunoanalysis on standard compact discs to determine low abundant compounds. Anal Chem 79:7628–7635
Noroozi Z, Kido H, Micic M, Pan H, Bartolome C, Princevac M, Zoval J, Madou M (2009) Reciprocating flow-based centrifugal microfluidics mixer. Rev Sci Instrum 80:075102-8
Press W, Teukolsky S, Vetterling W, Flannery B (2007) Numerical recipes: the art of scientific computing. Cambridge University Press, Cambridge
Siegrist J, Gorkin R, Clime L, Roy E, Peytavi R, Kido H, Bergeron M, Veres T, Madou M (2009) Serial siphon valving for centrifugal microfluidic platforms. Microfluid Nanofluid. doi:10.1007/s10404-009-0523-5
Steigert J, Brenner T, Grumann M, Riegger L, Lutz S, Zengerle R, Ducrée J (2007) Integrated siphon-based metering and sedimentation of whole blood on a hydrophilic lab-on-a-disk. Biomed Microdevices 9:675–679
Zoval JV, Madou MJ (2004) Centrifuge-based fluidic platforms. Proc IEEE 92:140–153
Acknowledgments
The authors wish to thank Will Southard and Kameel Abi-Samra for their support during CD fabrication.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
(WMV 8,014 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gorkin, R., Clime, L., Madou, M. et al. Pneumatic pumping in centrifugal microfluidic platforms. Microfluid Nanofluid 9, 541–549 (2010). https://doi.org/10.1007/s10404-010-0571-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-010-0571-x