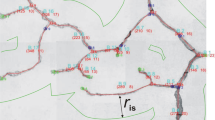
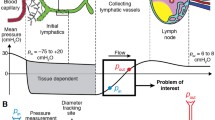
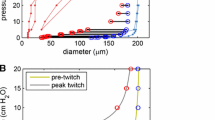
Abstract
Our published model of a lymphatic vessel consisting of multiple actively contracting segments between non-return valves has been further developed by the incorporation of properties derived from observations and measurements of rat mesenteric vessels. These included (1) a refractory period between contractions, (2) a highly nonlinear form for the passive part of the pressure–diameter relationship, (3) hysteretic and transmural-pressure-dependent valve opening and closing pressure thresholds and (4) dependence of active tension on muscle length as reflected in local diameter. Experimentally, lymphatic valves are known to be biased to stay open. In consequence, in the improved model, vessel pumping of fluid suffers losses by regurgitation, and valve closure is dependent on backflow first causing an adverse valve pressure drop sufficient to reach the closure threshold. The assumed resistance of an open valve therefore becomes a critical parameter, and experiments to measure this quantity are reported here. However, incorporating this parameter value, along with other parameter values based on existing measurements, led to ineffective pumping. It is argued that the published measurements of valve-closing pressure threshold overestimate this quantity owing to neglect of micro-pipette resistance. An estimate is made of the extent of the possible resulting error. Correcting by this amount, the pumping performance is improved, but still very inefficient unless the open-valve resistance is also increased beyond the measured level. Arguments are given as to why this is justified, and other areas where experimental data are lacking are identified. The model is capable of future adaptation as new experimental data appear.















Similar content being viewed by others
Notes
A further term describes valve failure by prolapse at a large negative value of \(\Delta p_{\mathrm{V}}\), but that capability is not exercised in the results described in this paper.
Recall that \(\Delta p_{\mathrm{tm}}=f_{\mathrm{passive}}(D)+f_{\mathrm{active}}(D,t)\), where \(f_{\mathrm{active}}(D,t) = 2M_{\mathrm{t}}(t)/D\) or \(2M(D,t)/D\).
In principle, an estimate of the magnitude of the artefact could be made using the Poiseuille equation, plus longitudinal measurements of pipette and vessel diameter, along with volume flow rate prior to valve closure, but flow rate was not measured by Davis et al. (2011).
References
Bertram CD (2012) Lumped-parameter modelling of microlymphatic vessels. Paper presented at the ECI conference, computational fluid dynamics in medicine and biology, Ein Bokek, Dead Sea, Israel, 25–30 March 2012
Bertram CD, Macaskill C, Moore JE jr (2011a) Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. ASME J Biomech Eng 133(1): 011008-1–011008-10. doi:10.1115/1.4002799
Bertram CD, Macaskill C, Moore JE jr (2011b) Effects of lymphangion subdivision in a numerical model of a lymphatic vessel. Paper presented at the ASME 2011 summer bioengineering conference (SBC2011) Farmington, Pennsylvania, USA 22–25 June 2011
Bertram CD, Macaskill C, Moore JE jr (2013) Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Eng (publ on-line 6 Feb). doi:10.1080/10255842.2012.753066
Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE jr (2011) Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol (Heart Circ Physiol) 301(1):H48–H60. doi:10.1152/ajpheart.00133.2011
Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC (2012) Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. American Journal of Physiology (Heart and Circulatory Physiology) 303(7):H795–H808. doi:10.1152/ajpheart.01097.2011
Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE jr., Zawieja DC (2006) Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13(7):597–610. doi:10.1080/10739680600893909
Gashev AA, Davis MJ, Zawieja DC (2002) Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540(Pt. 3):1023–1037
Gashev AA, Davis MJ, Delp MD, Zawieja DC (2004) Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11(6):477–492
Imtiaz MS, von der Weid PY, van Helden DF (2010) Synchronization of \(\text{ Ca }^{2+}\) oscillations: a coupled oscillator-based mechanism in smooth muscle. FEBS J 277(2):278–285. doi:10.1111/j.1742-4658.2009.07437.x
Mazzoni MC, Skalak TC, Schmid-Schönbein GW (1987) Structure of lymphatic valves in the spinotrapezius muscle of the rat. Blood Vessels 24(6):304–312
Peng H, Matchkov V, Ivarsen A, Aalkjær C, Nilsson H (2001) Hypothesis for the initiation of vasomotion. Circ Res 88(8):810–815. doi:10.1161/hh0801.089603
Rahbar E, Moore JE jr. (2011) A model of a radially expanding and contracting lymphangion. J Biomech 44(6):1001–1007. doi:10.1016/j.jbiomech.2011.02.018
Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ (2012) Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol (Heart and Circulatory Physiology) 303(7):H809–H824. doi:10.1152/ajpheart.01098.2011
Schmid-Schönbein GW (1990) Microlymphatics and lymph flow. Physiol Rev 70(4):987–1028
Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ (1993) Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol (Heart Circ Physiol) 264((4, Pt. 2)):H1283–H1291
Zhang RZ, Gashev AA, Zawieja DC, Davis MJ (2007) Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol (Heart Circ Physiol) 292(4):H1943–H1952
Acknowledgments
CDB is grateful for facilities afforded him during a sabbatical year at the Laboratoire d’Hydrodynamique, Ecole Polytechnique, France. MJD’s laboratory was supported by US National Institutes of Health grant R01-HL-089784, and JEM’s by grant R01-HL-094269. JEM gratefully acknowledges support from the Royal Academy of Engineering and a Royal Society-Wolfson Research Merit Award.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The passive constitutive relation is a nonlinear function \(\Delta p_{\mathrm{tm}} = f_{\mathrm{p}}(D)\), where
When \(P_{\mathrm{d}}\) defines the slope \(4P_{\mathrm{d}}/D_{\mathrm{d}}\) of the function at \(\Delta p_{\mathrm{tm}} = 0\), the constants have the values \(c_{1} = -2.34457751, c_{2} = 1.1262924, c_{3} = 3.76013762, c_{4} = 79.991135, c_{5} = 1.0028029, c_{6} = 1.59133174, c_{7} = 3.69692633, c_{8} = 0.20699868, c_{10} = -0.0180867408\) and \(c_{11}\) = 0.32538081. This system is convenient in the numerical model; however, a different scaling system is preferred in experiments. The distension-limiting region of the curve to the right of the knee is reached by \(\Delta p_{\mathrm{tm}} = 5\,\text{ cm }\;\text{ H }_{2}\)O; the corresponding diameter \(c_{9}\) is used to normalise the measurements of \(D\;(D_{\mathrm{d}}\) then takes the value \(c_{11})\). \(P_{\mathrm{d}}\) can be used to refer to this point if so desired (for the defining curve under consideration, it then takes the value 4,905 = 5 \(\times \) 981 dyn \(\text{ cm }^{-2}\)), in which case the values of \(c_{1}, c_{3}, c_{6}, c_{7}\) and \(c_{10}\) must be multiplied by 0.7464031.
The active length–tension relation for contraction is a nonlinear function \(M_{\mathrm{d}}(D)\), where
with \(D_{\mathrm{a}} = 0.85c_{9}, D_{\mathrm{b}} = \text{2c }_{9} \text{ and } s_{\mathrm{d}} = 3.25/D_\mathrm{d}\).
Rights and permissions
About this article
Cite this article
Bertram, C.D., Macaskill, C., Davis, M.J. et al. Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol 13, 401–416 (2014). https://doi.org/10.1007/s10237-013-0505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0505-0