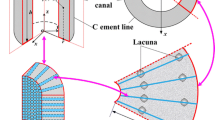
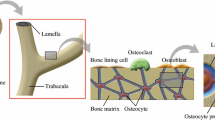
Abstract
An analytical model for the determination of the permeability in the lacunar–canalicular porosity of bone using cyclic loading is described in this contribution. The objective of the analysis presented is to relate the lacunar–canalicular permeability to a particular phase angle that is measurable when the bone is subjected to infinitesimal cyclic strain. The phase angle of interest is the lag angle between the applied strain and the resultant stress. Cyclic strain causes the interstitial fluid to move. This movement is essential for the viability of osteocytes and is believed to play a major role in the bone mechanotransduction mechanism. However, certain bone fluid flow properties, notably the permeability of the lacunar–canalicular porosity, are still not accurately determined. In this paper, formulas for the phase angle as a function of permeability for infinitesimal cyclic strain are presented and mathematical expressions for the storage modulus, loss modulus, and loss tangent are obtained. An accurate determination of the PLC permeability will improve our ability to understand mechanotransduction and mechanosensory mechanisms, which are fundamental to the understanding of how to treat osteoporosis, how to cope with microgravity in long-term manned space flights, and how to increase the longevity of prostheses that are implanted in bone tissue.
Similar content being viewed by others
Abbreviations
- \({\hat{A}_r},\, {\hat{A}_\theta},\, {\hat{A}_z}\) :
-
Three components of the 6D vector representing the Biot effective stress coefficient for PLC
- r :
-
Arbitrary radius of the osteon
- r o :
-
Outer radius of the osteon
- r i :
-
Inner radius of the osteon
- a :
-
Non-dimensional inner radius of the Osteon (a = r i / r o )
- \({\hat{{\bf C}}^{i}}\) :
-
(i = m, d, u) elasticity or stiffness matrix for the matrix material, drained elastic constants and undrained elastic constants (2nd order tensor in 6D)
- c :
-
Pore fluid pressure diffusion constant in the lacunar–canalicular porosity
- \({\hat{{\bf E}}}\) :
-
Strain, a vector in 6D, equivalent to the strain tensor in 3D
- E :
-
Modulus of elasticity
- f o :
-
Constant of integration determined in Eq.(16)
- I o , I 1 :
-
Modified Bessel functions of the first kind
- i :
-
Imaginary number \({\left(i=\sqrt{-1}\right)}\)
- K f :
-
Compressibility of the fluid
- \({K_{\rm Reff}^i}\) :
-
Reuss lower bound on the effective (isotropic) bulk modulus of the anisotropic elastic material (i = m, d)
- K o , K 1 :
-
Modified Bessel functions of the second kind
- K rr :
-
Radial permeability
- \({\tilde{p}(r,t)}\) :
-
Pore fluid pressure in the PLC
- \({\tilde{u}(r,t)}\) :
-
Displacement vector
- \({\varepsilon_o}\) :
-
Strain amplitude of the cyclic applied strain
- \({\phi}\) :
-
Porosity
- λ:
-
Ratio of r/r o (non-dimensional)
- ω :
-
Angular frequency associated with the cyclic loading
- \({\bar{\omega}}\) :
-
Dimensionless angular frequency
- μ :
-
Viscosity of the pore fluid
- ν ij :
-
Poisson’s ratios (i, j = r, θ, z)
- σ * :
-
Average resultant stress due the applied cyclic strain
- σ o :
-
Magnitude of the average resultant stress
- \({\Upsilon}\) :
-
Constant of dimension force
- Λ:
-
Solid–fluid compliance contrast coefficient
- \({\tilde{\zeta}(r,t)}\) :
-
Variation of fluid content and
- C * :
-
Dynamic elastic modulus
- δ :
-
Phase angle
- d :
-
The drained condition of the porous solid
- u :
-
The undrained condition of the porous solid
- f :
-
The fluid component
- m :
-
The matrix material of the porous solid
References
Abramowitz M, Stegun IA (1964) Handbook of mathematical functions with formulas, graphs and mathematical tables. Natl Bureau Standards Appl Math Ser 55: 379–380
Beno T, Yoon YJ, Cowin SC, Fritton SP (2006) Estimation of bone permeability using accurate microstructural measurements. J Biomech 39: 2378–2387
Buechner PM, Lakes RS (2003) Size effects in the elasticity and viscoelasticity of bone. Biomech Model Mechanobiol 1: 295–301
Burger EH, Klein-Nulend J, van der Plas A, Nijweide PJ (1995) Function of osteocytes in bone—their role in mechanotransduction. J Nutr 125(7 Suppl): 2020S–2023S
Ciani C, Ramirez Marin PAR, Doty SB, Fritton SP (2007) Bone microstructure in OVX and normal rat bone as revealed by confocal and electron microscopy. In: Bioengineering conference, 2007. NEBC ’07. IEEE 33rd annual Northeast, pp 23–24
Cowin SC (1999) Bone poroelasticity. J Biomech 32: 217–238
Cowin SC (2001) Bone Mechanics Handbook, vol 17, 2nd edn. CRS Press, Boca Raton, pp 2–5
Cowin SC, Doty SB (2007) Tissue mechanics. Springer, Berlin
Cowin SC, Gailani G, Benalla M (2009) Hierarchical poroelasticity: movement of interstitial fluid between porosity levels in bones. Philos Trans R Soc 367: 3401–3443
Cowin SC, Moss-Salentijn L, Moss ML (1991) Candidates for the mechanosensory system in bone. J Biomech Eng 113: 191–197
Cowin SC, Weinbaum S, Zeng Y (1995) A case for bone canaliculi as the anatomical site of strain-generated potentials. J Biomech 28: 1281–1296
Drabousky DP (2009) Prony series representation and interconversion of viscoelastic material functions of equine cortical bone. Department of Mechanical and Aerospace Engineering, Western Reserve University, Cleveland
Fornells P, García-Aznar JM, Doblaré M (2007) A finite element dual porosity approach to model deformation-induced fluid flow in cortical bone. Ann Biomed Eng 35: 1687–1698
Fritton SP, Wang L, Weinbaum S, Cowin SC (2001) Interaction of mechanical loading, blood flow, and interstitial fluid flow in osteonal bone. Proc Bioeng Conf BED 50: 341–342
Fritton SP, Weinbaum S (2009) Fluid and solute transport in bone: flow induced mechanotransduction. Annu Rev Fluid Mech 41: 347–374
Gailani GB, Benalla M, Mahamud R, Cowin SC, Cardoso L (2009) Experimental determination of the permeability in the lacunar-canalicular porosity of bone. J Biomech Eng 131: 1010071–1010077
Gailani GB, Cowin SC (2008) The unconfined compression of a poroelastic annular cylindrical disk. Mech Mater 40: 507–523
Gardinier JD, Townend CW, Jen KP, Wu Q, Duncan RL, Wang L (2010) In situ permeability measurement of the mammalian lacunar-canalicular system. J Bone 46: 1075–1081
Garner E, Lakes R, Lee T, Swan C, Brand R (2000) Viscoelastic dissipation in compact bone: implications for stress-Induced fluid flow in bone. J Biomech Eng 122: 167–172
Goulet GC, Coombe D, Martinuzzi RJ, Zernicke RF (2009) Poroelastic evaluation of fluid movement through the lacunocanalicular system. Ann Biomed Eng 37(7): 1390–1402
Gururaja S, Kim HJ, Swan CC, Brand RA, Lakes RS (2005) Modeling deformation-induced fluid flow in cortical bone’s canalicular-lacunar system. Ann Biomed Eng 33: 7–25
Han Y, Cowin SC, Schaffler MB, Weinbaum S (2004) Mechanotransduction and strain amplification in osteocyte cell processes and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA 101: 16689–16694
Jee W (1988) The skeletal tissues. In: Weiss L. (eds) Cell and tissue biology: a textbook of histology. Urban & Schwarzenberg, Baltimore, p 207
Kameo Y, Adachi T, Sato N, Hojo M (2010) Estimation of bone permeability considering the morphology of lacuno-canalicular porosity. J Mech Behav Biomed Mater 3: 240–248
Lakes RS (1982) Dynamical study of couple stress effects in human compact bone. J Biomech Eng 104: 7–11
Nguyen V, Lemaire T, Naili S (2009) Numerical study of deformation-induced fluid flows in periodic osteonal matrix under harmonic axial loading. C R Mecanique 337: 268–276
Oyen ML (2008) Poroelastic nanoindentation responses of hydrated bone. J Mater Res 23: 1307–1314
Ramtani S (2007) Parametric sensitivity analysis applied to a specific one-dimensional internal bone remodeling problem. Comput Biol Med 37: 1203–1209
Rémond A, Naïli S, Lemaire T (2008) Interstitial fluid flow in the osteon with spatial gradients of mechanical properties: a finite element study. Biomech Model Mechanobiol 7: 487–495
Smit TH, Huyghe JM, Cowin SC (2002) Estimation of the poroelastic parameters of bone. J Biomech 35: 829–836
Swan CC, Lakes RS, Brand RA, Stewart KJ (2003) Micromechanically based poroelastic modeling of fluid flow in Haversian bone. J Biomech Eng 125: 25–37
Wang L, Fritton SP, Cowin SC, Weinbaum S (1999) Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J Biomech 32: 663–672
Wang L, Fritton SP, Weinbaum S, Cowin SC (2003) On bone adaptation due to venous stasis. J Biomech 36: 1439–1451
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27: 339–360
Wu D, Ci S, Luo H (2011) A theoretical framework for interaction measure and sensitivity analysis in cross-layer design. ACM Trans Model Comput Simulat 21: 1–26
Yang G, Kabel J, Rietbergen BV, Odgaard A, Huiskes R, Cowin SC (1999) The anisotropic Hooke’s law for cancellous bone and wood. J Elast 53: 125–146
Zeng Y, Cowin SC, Weinbaum S (1994) A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng 22: 280–292
Zhang D, Weinbaum S, Cowin SC (1998) Estimates of the peak pressures in the bone pore water. J Biomech Eng 120: 697–703
Zhou X, Novotny JE, Wang L (2008) Modeling fluorescence recovery after photobleaching in loaded bone: potential applications in measuring fluid and solute transport in the osteocytic lacunar-canalicular system. Ann Biomed Eng 36: 1961–1977
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benalla, M., Cardoso, L. & Cowin, S.C. Analytical basis for the determination of the lacunar–canalicular permeability of bone using cyclic loading. Biomech Model Mechanobiol 11, 767–780 (2012). https://doi.org/10.1007/s10237-011-0350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0350-y