Abstract
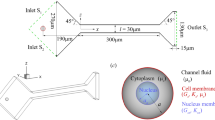
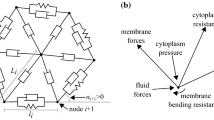
Cell entry into a micro-channel has potential applications in cell sorting and cancer diagnostics. In this paper, we numerically model breast cancer cell entry into a constricted micro-channel. Our results indicate that the cell velocity decreases during entry and increases after entry, an observation in agreement with experiments. We found that the cell entry time depend strongly on the cortical stiffness and is minimum at some critical cortical elasticity. In addition, we found that for the same entry time, a stiff nucleus is displaced toward the cell front, whereas a viscous nucleus is displaced toward the rear. In comparison, the nucleus is less sensitive to the viscosity of the cytoplasm. These observations suggest that specific intra-cellular properties can be deduced non-invasively during cell entry, through the inspection of the nucleus using suitable illumination techniques, such as fluorescent labeling.
Similar content being viewed by others
References
Agresar G, Linderman J, Tryggvason G, Powell K (1998) An adaptive, cartesian, front-tracking method for the motion, deformation and adhesion of circulating cells. J Comp Phys 143: 346–380
Bathe M, Shirai A, Doerschuk C, Kamm R (2002) Neutrophil transit times through pulmonary capillaries: the effects of capillary geometry and fmlp-stimulation. Biophys J 83: 1917–1933
Diaz A, Barthes-Biesel D (2002) Entrance of a bioartificial capsule in a pore. Comp Mod Eng Sci 3(3): 321–337
Doerschuk C, Beyers N, Coxson H, Wiggs B, Hogg J (1993) Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol 74: 3040–3045
Dong C, Skalak R (1992) Leukocyte deformability: finite element modeling of large viscoelastic deformation. J Theo Bio 158(2): 173–193
Dong C, Skalak R, Sung K (1991) Cytoplasmic rheology of passive neutrophils. Biorheol 28: 557–567
Drury J, Dembo M (2001) Aspiration of human neutrophils: effects of shear thinning and cortical dissipation. Biophys J 81: 3166–3177
Evans E, Yeung A (1989) Apparent viscosity and cortical tension of blood granulocytes determined by micropipette aspiration. Biophys J 56: 151–160
Faivre M (2006) Drops, vesicles and red blood cells: deformability and behavior under flow. Université Joseph Fourier, Ph.D. Thesis
Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson H, Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, BilbyGuck C (2005) Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88: 3689–3698
Hochmuth R, Ting-Beall H, Beaty B, Needham D, Tran-Son-Tay R (1993) Viscosity of passive human neutrophils undergoing small deformations. Biophys J 64: 1596–1601
Hou H, Li Q, Lee G, Kumar A, Ong C, Lim C (2009) Deformability study of breast cancer cells using microfluidics. Biomed Microdevices 11: 557–564
Kamm R (2002) Cellular fluid mechanics. Ann Rev Fluid Mech 34: 211–232
Kan H, Udaykumar H, Shyy W, Tran-Son-Tay R (1998) Hydrodynamics of a compound drop with application to leukocyte modeling. Phys Fluids 10: 760–774
Kan H, Shyy W, Udaykumar H, Vigneron P, Tran-Son-Tay R (1999) Effects of nucleus on leukocyte recovery. Ann Biomed Eng 27: 648–655
Khismatullin D, Truskey G (2005) Three dimensional numerical simulation of receptor-mediated leukocyte adhesion to surfaces: effects of cell deformability and viscoelasticity. Phys Fluids 17: 031,505
Lee G, Lim C (2007) Biomechanics approaches to studying human diseases. Trends Biotech 25: 111–118
Lim C, Zhou E, Quek S (2006) Mechanical models for living cells—a review. J Biomech 39: 195–216
Lincoln B, Erickson H, Schinkinger S, Wottawah F, Mitchell D, Ulvick S, Bilby C, Guck J (2004) Deformability-based flow cytometry. Cytometry A 59(2): 203–209
Marella S, Udaykumar H (2004) Computational analysis of the deformability of leukocytes modeled with viscous and elastic structural components. Phys Fluids 16: 244–264
McDonald J, Duffy D, Anderson J, Chiu D, Wu H, Schueller O, Whitesides G (2000) Fabrication of microfluidic systems in poly-dimethylsiloxane. Electrophoresis 21: 27–40
N’Dri N, Shyy W, Tran-Son-Tay R (2003) Computational modeling of cell adhesion and movement using a continuum-kinetics approach. Biophys J 85: 2273–2286
Needham D, Hochmuth R (1990) Rapid flow of passive neutrophils into a 4 μm pipette and measurement of cytoplasmic viscosity. J Biomech Eng 112: 269–276
Needham D, Hochmuth R (1992) A sensitive measure of surface stress in the resting neutrophil. Biophys J 61: 1664–1670
Peskin C (1977) Numerical analysis of blood flow in the heart. J Comp Phys 25: 220–252
Peskin C (2002) The immersed boundary method. Acta Numerica 11: 479–517
Quéguiner C, Barthès-Biesel D (1997) Axisymmetric motion of capsules through cylindrical channels. J Fluid Mech 348: 349
Schmid-Schönbein G, Shih Y, Chien S (1980) Morphometry of human leukocytes. Blood 56: 866
Schmid-Schönbein G, Sung K, Tozeren H, Skalak R, Chien S (1981) Passive mechanical properties of human leukocytes. Biophys J 36: 243–256
Secomb T, Hsu R (1996) Analysis of red blood cell motion through cylindrical micropores: effects of cell properties. Biophys J 71: 1095–1101
Secomb T, Hsu R (1996) Motion of red blood cells in capillaries with variable cross-sections. J Biomech Eng 118: 538–544
Shyy W, Francois M, Udaykumar H, N’Dri N, Trans-Son-Tay R (2001) Moving boundaries in micro-scale biofluid dynamics. Appl Mech Rev 54: 405–453
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomat 3: 413–438
Tran-Son-Tay R, Kan H, Udaykumar H, Damay E, Shyy W (1998) Rheological modeling of leukocytes. Med Biol Eng Comp 36: 246–250
Tsai M, Frank R, Waugh R (1993) Passive mechanical behavior of human neutrophils: power-law fluid. Biophys J 65: 2078–2088
Tseng Y, Wirtz D (2001) Mechanics and multiple-particle tracking micro-heterogeneity of α-actinin-cross-linked actin filament networks. Biophys J 81: 1643–1656
Udaykumar H, Kan H, Shyy W, Tran-Son-Tay R (1997) Multiphase dynamics in arbitrary geometries on fixed cartesian grids. J Comp Phys 137: 366–405
Valberg P, Albertini D (1985) Cytoplasmic motions, rheology, and structure probed by a novel magnetic particle method. J Cell Bio 101: 130–140
Yap B, Kamm R (2005) Mechanical deformation of neutrophils into narrow channels induces pseudopod projection and changes in biomechanical properties. J Appl Physiol 98: 1930–1939
Yeung A, Evans E (1989) Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipettes. Biophys J 56: 139–149
Yue P, Feng J, Shen J (2004) A diffuse-interface method for simulating two-phase flows of complex fluids. J Fluid Mech 515: 293–317
Zhou C, Yue P, Feng J (2007) Simulation of neutrophil deformation and transport in capillaries using newtonian and viscoelastic drop models. Ann Biomed Eng 35: 776–780
Zhou C, Yue P, Feng J (2008) Deformation of a compound drop through a contraction in a pressure-driven pipe flow. Int J Multiphase Flow 34: 102–109
Zhou D, Yue P, Feng J (2008) Visco-elastic effects on drop deformation in a converging pipe flow. J Rheol 32: 469–487
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leong, F.Y., Li, Q., Lim, C.T. et al. Modeling cell entry into a micro-channel. Biomech Model Mechanobiol 10, 755–766 (2011). https://doi.org/10.1007/s10237-010-0271-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0271-1