Abstract
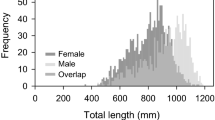
We herein evaluate several reproductive metrics of Hawaiian Archipelagic populations of the bluespine unicornfish (Naso unicornis), an economically and ecologically important, broadly distributed tropical Pacific reef fish, based on multi-year, fishery-dependent and fishery-independent collections. Sex-specific spawning seasonality was characterized for fish collected mostly from Oahu (Main Hawaiian Islands, MHI) using a gonadosomatic index. Histological slides preparations were used to score gonad developmental phase and to classify individuals of either sex as immature or mature. Sex-specific median body lengths at maturity (L50) were estimated by logistic fits of proportion mature versus length class. Spawning was highly seasonal in Hawaii, with a single brief (May–June) peak spawning period. Proportionate gonad-to-body weight values were relatively low, averaging only about 0.1 % and 0.6 % across all months of year and 0.16 % and 1.03 % during May–June for males and females, respectively. Median lengths at sexual maturity differed between the sexes. L50 values for fish collected throughout all months of year were 30.1 ± 0.5 (standard error) cm Fork Length (FL) for males and 35.5 ± 0.7 cm FL for females. Spawning seasonality and L50 estimates for bluespine unicornfish in Hawaii suggest that the species spawns several months earlier in the calendar year and matures at larger body lengths in Hawaii versus Guam, the Northern Mariana Islands, and Pohnpei in the Federated States of Micronesia. Estimated lengths at sexual maturity are compared to the minimum length (14 inches or 35.6 cm FL) mandated for this species in Hawaii: median size at maturity occurs at a length appreciably less than (males) or approximately equal to (females) minimum legal size. A likely disproportionately large contribution of old females to population replenishment is discussed relative to the minimum size limit.






Similar content being viewed by others
References
Andrews AH, DeMartini EE, Brodziak J, Nichols RS, Humphreys RL (2012) A long-lived life history for a tropical deepwater snapper (Pristipomoides filamentosus): bomb-radiocarbon and lead-radium dating as extensions of daily increment analyses in otoliths. Can J Fish Aquat Sci 69:1850–1869
Bejarano S, Golbuu Y, Sapolu T, Mumby PJ (2013) Ecological risk and the exploitation of herbivorous reef fish across Micronesia. Mar Ecol Prog Ser 482:197–215
Berkeley SA, Hixon MA, Larson RJ, Love MS (2004) Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisher 29:23–32
Birkeland C, Dayton PK (2005) The importance in fisheries management of leaving the big ones. Trends Ecol Evol 20:356–358
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish: Dynam Manag Ecosystem Sci 3:52–70
Bushnell ME, Claisse JT, Laidley CW (2010) Lunar and seasonal patterns in fecundity of an indeterminate, multiple-spawning surgeonfish, the yellow tang Zebrasoma flavescens. J Fish Biol 76:1343–1361
Choat JH, Axe L (1996) Growth and longevity in acanthurid fishes: an analysis of otolith increments. Mar Ecol Prog Ser 134:15–26
Choat JH, Robertson DR (2002) Age-based studies. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, Amsterdam, pp 57–80
Claisse JT, Kienzle M, Bushnell ME, Shafer DJ, Parrish JD (2009) Habitat- and sex-specific life history patterns of yellow tang Zebrasoma flavescens in Hawaii, USA. Mar Ecol Prog Ser 389:245–255
DeMello JK (2004) Commercial marine landings from fisheries on the coral reef ecosystem of the Hawaiian Archipelago. In: Friedlander AM (ed) Status of Hawaii’s coastal fisheries in the New Millenium; Honolulu, 01 November. Am Fish Soc, Hawaii Chapter, pp 157–170
Donovan MK, Friedlander AM, DeMartini EE, Donahue M, Williams ID (2012) Demographic patterns in the peacock grouper (Cephalopholis argus), an introduced Hawaiian reef fish. Environ Biol Fish 96:981–994
Eble JA, Langston R, Bowen BW (2009) Growth and reproduction of Hawaiian kala, Naso unicornis. Final report prepared for fisheries local action strategy. Division of Aquatic Resources, Department of Land and Natural Resources, Hawaii
Fishelson L, Montgomery LW, Myrberg AH Jr (1987) Biology of surgeonfish Acanthurus nigrofuscus with emphasis on changeover in diet and annual gonadal cycles. Mar Ecol Prog Ser 39:37–47
Hoey AS, Bellwood DR (2009) Limited functional redundancy in a high diversity system: single species dominates key ecological process on coral reefs. Ecosystems 12:1316–1328
Houk P, Rhodes K, Cuetos-Bueno J, Lindfield S, Fread V, McIlwain JL (2012) Commercial coral-reef fisheries across Micronesia: a need for improving management. Coral Reefs 31:13–26
Jones RS (1968) Ecological relationships in Hawaiian and Johnston Island Acanthuridae (surgeonfishes). Micronesica 4:309–361
Lieske E, Myers R (1994) Collins pocket guide. Coral reef fishes: Caribbean, Indian Ocean and Pacific Ocean including the Red Sea. HarperCollins Publishers, London
Marshell A, Mills JS, Rhodes KL, McIlwain J (2011) Passive acoustic telemetry reveals highly variable home range and movement patterns among unicornfish within a marine reserve. Coral Reefs 30:631–642
Meyer CG, Holland KN (2005) Movement patterns, home range size and habitat utilization of the bluespine unicornfish, Naso unicornis (Acanthuridae) in a Hawaiian marine reserve. Environ Biol Fish 73:201–210
Montgomery WL, Galzin R (1993) Seasonality in gonads, fat deposits and condition of tropical surgeonfishes (Teleostei: Acanthuridae). Mar Biol 115:529–536
Montgomery WL, Umino T, Nakagawa H, Vaughn I, Shibuno T (1999) Lipid storage and composition in tropical surgeonfishes (Teleostei: Acanthuridae). Mar Biol 133:137–144
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge Univ Press, Cambridge
Randall JE (2007) Reef and Shore Fishes of the Hawaiian Islands. Sea Grant College Program, Univ Hawaii, Hawaii
Robertson DR (1983) On the spawning behavior and spawning cycles of eight surgeonfishes (Acanthuridae) from the Indo-Pacific. Environ Biol Fish 9:193–223
Robertson DR (1985) Sexual size dimorphism in surgeon fishes. Proc 5th Internat Coral Reef Congress, Tahiti 5:403–408
Santelices B (1977) Water movement and seasonal algal growth in Hawaii. Mar Biol 43:225–235
SAS Institute, Inc (2006) Base SAS 9.1.3 Procedures Guide. 2nd ed, Vols 1–4. SAS Institute, Inc, Cary, North Carolina
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth and maturation in teleosts. Amer Zool 21:342–343
Acknowledgments
We greatly appreciate assistance provided by numerous students and staff of the Hawaii Institute of Marine Biology (HIMB), the floor workers at Tamashiro Market, and G. Ishimoto of Diamond Head Seafood, Honolulu, in obtaining specimens. The crew of the NOAA ship Hi`ialakai, staff at the Papahonaumokuakea Marine National Monument, and the Hawaii Department of Land and Natural Resources facilitated collections made within the Northwestern Hawaiian Islands. We also thank D. Krupp and Windward Community College for providing laboratory space; HIMB for small craft access; numerous staff of the Pacific Islands Fisheries Science Center, Life History Program, for assistance with laboratory fish processing; K. Rhodes for discussion of bluespine unicornfish reproductive data from Pohnpei and Guam; and R. Humphreys and M. Sundberg for constructive comments on a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
DeMartini, E.E., Langston, R.C. & Eble, J.A. Spawning seasonality and body sizes at sexual maturity in the bluespine unicornfish, Naso unicornis (Acanthuridae). Ichthyol Res 61, 243–251 (2014). https://doi.org/10.1007/s10228-014-0393-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-014-0393-z