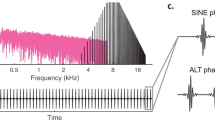
Abstract
The acoustic change complex (ACC) is a scalp-recorded cortical evoked potential complex generated in response to changes (e.g., frequency, amplitude) in an auditory stimulus. The ACC has been well studied in humans, but to our knowledge, no animal model has been evaluated. In particular, it was not known whether the ACC could be recorded under the conditions of sedation that likely would be necessary for recordings from animals. For that reason, we tested the feasibility of recording ACC from sedated cats in response to changes of frequency and amplitude of pure-tone stimuli. Cats were sedated with ketamine and acepromazine, and subdermal needle electrodes were used to record electroencephalographic (EEG) activity. Tones were presented from a small loudspeaker located near the right ear. Continuous tones alternated at 500-ms intervals between two frequencies or two levels. Neurometric functions were created by recording neural response amplitudes while systematically varying the magnitude of steps in frequency centered in octave frequency around 2, 4, 8, and 16 kHz, all at 75 dB SPL, or in decibel level around 75 dB SPL tested at 4 and 8 kHz. The ACC could be recorded readily under this ketamine/azepromazine sedation. In contrast, ACC could not be recorded reliably under any level of isoflurane anesthesia that was tested. The minimum frequency (expressed as Weber fractions (df/f)) or level steps (expressed in dB) needed to elicit ACC fell in the range of previous thresholds reported in animal psychophysical tests of discrimination. The success in recording ACC in sedated animals suggests that the ACC will be a useful tool for evaluation of other aspects of auditory acuity in normal hearing and, presumably, in electrical cochlear stimulation, especially for novel stimulation modes that are not yet feasible in humans.














Similar content being viewed by others
References
Arlinger SD, Jerlvall LB (1979) Results of psychoacoustic and cortical evoked potential experiments using frequency and amplitude modulated stimuli. Scand Audiol Suppl: 229–239
Arlinger SD, Jerlvall LB, Ahren T, Holmgren EC (1976) Slow evoked cortical responses to linear frequency ramps of a continuous pure tone. Acta Physiol Scand 98:412–424
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Brown CJ, Etler C, He S, O’Brien S, Erenberg S, Kim J-R, Dhuldhoya AN, Abbas PJ (2008) The electrically evoked auditory change complex: preliminary results from nucleus cochlear implant users. Ear Hear 29:704–717
Brown M, Irvine DR, Park VN (2004) Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex 14:952–965
Butler RA, Diamond IT, Neff WD (1957) Role of auditory cortex in discrimination of changes in frequency. J Neurophysiol 20:108–120
Chen KH, Small SA (2015) Elicitation of the acoustic change complex to long-duration speech stimuli in four-month-old infants. Int J Otolaryngol 2015:562030
Dimitrijevic A, Michalewski HJ, Zeng F-G, Pratt H, Starr A (2008) Frequency changes in a continuous tone: auditory cortical potentials. Clin Neurophysiol 119:2111–2124
Dimitrijevic A, Lolli B, Michalewski HJ, Pratt H, Zeng F-G, Starr A (2009) Intensity changes in a continuous tone: auditory cortical potentials comparison with frequency changes. Clin Neurophysiol 120:374–383
Dimitrijevic A, Starr A, Bhatt S, Michalewski HJ, Zeng FG, Pratt H (2011) Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol 122:594–604
Efron B, Tibshirani R (1991) Statistical data analysis in the computer age. Science 253:390–395
Elliott DN, McGee TM (1965) Effect of cochlear lesions upon audiograms and intensity discrimination in cats. Ann Otol Rhinol Laryngol 74:386–408
Elliott DN, Stein L, Harrison MJ (1960) Determination of absolute-intensity thresholds and frequency-difference thresholds in cats. J Acoust Soc Am 32:380–384
Green DM, Swets JA (1966) Signal detection theory and psychophysics. John Wiley & Sons, Inc, New York, NY
Haenggi M, Ypparila H, Takala J, Korhonen I, Luginbuhl M, Petersen-Felix S, Jakob SM (2004) Measuring depth of sedation with auditory evoked potentials during controlled infusion of propofol and remifentanil in healthy volunteers. Anesth Analg 99:1728–1736
Hall JL, Goldstein Jr MH (1968) Representation of binaural stimuli by single units in primary auditory cortex of unanesthetized cats. J Acoust Soc Am 43:456–461
Han JH, Dimitrijevic A (2015) Acoustic change responses to amplitude modulation: a method to quantify cortical temporal processing and hemispheric asymmetry. Front Neurosci 9:38
Harris KC, Mills JH, Dubno JR (2007) Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear Res 228:58–68
He S, Grose JH, Buchman CA (2012) Auditory discrimination: the relationship between psychophysical and electrophysiological measures. Int J Audiol 51:771–782
Heffner RS, Heffner HE (1985) Hearing range of the domestic cat. Hear Res 19:85–88
Hienz RD, Sachs MB, Aleszczyk CM (1993) Frequency discrimination in noise: comparison of cat performances with auditory-nerve models. J Acoust Soc Am 93:462–469
Hine J, Debener S (2007) Late auditory evoked potentials asymmetry revisited. Clin Neurophysiol 118:1274–1285
Igarashi M, Cranford JL, Allen EA, Alford BR (1979a) Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. V. Pure-tone intensity discrimination. Acta Otolaryngol 87:429–433
Igarashi M, Cranford JL, Nakai Y, Alford BR (1979b) Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. IV. Study on pure-tone frequency discrimination. Acta Otolaryngol 87:79–83
Kohn M, Lifshitz K, Litchfield D (1978) Averaged evoked potentials and frequency modulation. Electroencephalogr Clin Neurophysiol 45:236–243
Macmillan NA, Kaplan HL (1985) Detection theory analysis of group data: estimating sensitivity from average hit and false-alarm rates. Psychol Bull 98:185–199.
Macmillan NA, Creelman CD (2005) Detection theory: a user’s guide, 2nd edn. Lawrence Erlbaum Associates, Mahwah, New Jersey
Martin BA (2007) Can the acoustic change complex be recorded in an individual with a cochlear implant? Separating neural responses from cochlear implant artifact. J Am Acad Audiol 18:126–140
Martin BA, Boothroyd A (1999) Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear Hear 20:33–44
Martin BA, Boothroyd A (2000) Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J Acoust Soc Am 107:2155–2161
Martin BA, Boothroyd A, Ali D, Leach-Berth T (2010) Stimulus presentation strategies for eliciting the acoustic change complex: increasing efficiency. Ear Hear 31:356–366
Martinez AS, Eisenberg LS, Boothroyd A (2013) The acoustic change complex in young children with hearing loss: a preliminary study. Semin Hear 34:278–287
Middlebrooks JC, Snyder RL (2007) Auditory prosthesis with a penetrating nerve array. J Assoc Res Otolaryngol 8:258–279
Middlebrooks JC, Snyder RL (2008) Intraneural stimulation for auditory prosthesis: modiolar trunk and intracranial stimulation sites. Hear Res 242:52–63
Middlebrooks JC, Snyder RL (2010) Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci 30:1937–1946
Moller AR, Jannetta PJ, Sekhar LN (1988) Contributions from the auditory nerve to the brain-stem auditory evoked potentials (BAEPs): results of intracranial recording in man. Electroencephalogr Clin Neurophysiol 71:198–211
Näätänen R, Gaillard AWK, Mäntysalo S (1978) Early selective-attention effect on evoked potential reinterpreted. Acta Psychol 42:313–329
Neff WD, Hind JE (1955) Auditory thresholds of the cat. J Acoust Soc Am 27:480–483
Nelken I, Ulanovsky N (2007) Mismatch negativity and stimulus-specific adaptation in animal models. J Psychophysiol 21:214–223.
Pantev C, Lutkenhoner B, Hoke M, Lehnertz K (1986) Comparison between simultaneously recorded auditory-evoked magnetic fields and potentials elicited by ipsilateral, contralateral and binaural tone burst stimulation. Audiology 25:54–61
Ross B, Herdman AT, Pantev C (2005) Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb Cortex 15:2029–2039
Savoia G, Esposito C, Belfiore F, Amantea B, Cuocolo R (1988) Propofol infusion and auditory evoked potentials. Anaesthesia 43(Suppl):46–49
Scheperle RA, Abbas PJ (2015) Peripheral and central contributions to cortical responses in cochlear implant users. Ear Hear 36:430–440
Sokolovski A (1973) Normal threshold of hearing for cat for free-field listening. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 203:232–240
Stecker GC, Harrington IA, Middlebrooks JC (2005) Location coding by opponent neural populations in the auditory cortex. PLoS Biol 3:e78
Thornton C, Catley DM, Jordan C, Lehane JR, Royston D, Jones JG (1983) Enflurane anaesthesia causes graded changes in the brainstem and early cortical auditory evoked response in man. Br J Anaesth 55:479–486
Tietze G, Afontshenko V (1978) The acoustically evoked potentials in the case of stimulation by frequency modulation (FM) near the hearing threshold, compared with tone burst stimulation. Scand Audiol 7:33–38
Yingling CD, Nethercut GE (1983) Evoked responses to frequency shifted tones: tonotopic and contextual determinants. Int J Neurosci 22:107–118
Acknowledgements
This work was supported by the National Institute on Deafness and Other Communication Disorders grants R01 DC000420 (to JCM) and T32 DC010775 (to AP). We thank Elizabeth A. McGuire and Zekiye Onsan for their technical and administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Presacco, A., Middlebrooks, J.C. Tone-Evoked Acoustic Change Complex (ACC) Recorded in a Sedated Animal Model. JARO 19, 451–466 (2018). https://doi.org/10.1007/s10162-018-0673-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-018-0673-9