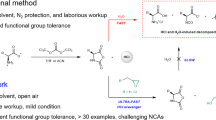
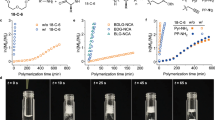
Abstract
Polypeptides have been widely utilized in the fields of biomaterials and biomedicine. Ever since N-carboxyanhydride (NCA) was reported by Hermann Leuchs in 1906, ring-opening polymerization of NCAs has been extensively used to prepare polypeptides. Despite continuous innovations, it is still challenging to synthesize polypeptides in high molecular weight efficiently. To address this challenge, we developed KHMDS/NaHMDS initiated fast NCA polymerization that is also moisture tolerant, open-flask amenable and terminal tunable. This NCA polymerization was able to proceed in most common solvents and meet the solubility requirement of variable NCA monomers and corresponding polypeptides. KHMDS can initiate γ-benzyl-L-glutamate-N-carboxyanhydride (BLG NCA) polymerization in a reaction rate 92 times faster than does hexylamine and 80 times faster than does triethylamine. This NCA polymerization also demonstrated easy and fast synthesis of gram-scale long chain polypeptides in an open flask.
Similar content being viewed by others
References
Liu, R.; Chen, X.; Gellman, S. H.; Masters, K. S. Nylon-3 polymers that enable selective culture of endothelial cells. J. Am. Chem. Soc. 2013, 135, 16296–16299.
Zhang, K.; Yan, S.; Li, G.; Cui, L.; Yin, J. In-situ birth of MSCs multicellular spheroids in poly(L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials 2015, 71, 24–34.
Liu, T.; Zhang, Y. F.; Liu, S. Y. Drug and plasmid DNA co-delivery nanocarriers based on abctype polypeptide hybrid miktoarm star copolymers. Chinese J. Polym. Sci. 2013, 31, 924–937.
Liu, H.; Xiao, Y.; Xu, H.; Guan, Y.; Zhang, J.; Lang, M. Reversible thermo-sensitivity induced from varying the hydrogen bonding between the side residues of rationally designed polypeptides. Chem. Commun. 2015, 51, 10174–10177.
Guo, A.; Yang, W.; Yang, F.; Yu, R.; Wu, Y. Well-defined poly(γ-benzyl-L-glutamate)-g-polytetrahydrofuran: synthesis, characterization, and properties. Macromolecules 2014, 47, 5450–5461.
Herce, H. D.; Schumacher, D.; Schneider, A. F. L.; Ludwig, A. K.; Mann, F. A.; Fillies, M.; Kasper, M. A.; Reinke, S.; Krause, E.; Leonhardt, H.; Cardoso, M. C.; Hackenberger, C. P. R. Cell-permeable nanobodies for targeted immunolabelling and antigen manipulation in living cells. Nat. Chem. 2017, 9, 762–771.
Lee, M. W.; Han, M.; Bossa, G. V.; Snell, C.; Song, Z.; Tang, H.; Yin, L.; Cheng, J.; May, S.; Luijten, E.; Wong, G. C. Interactions between membranes and “metaphilic” polypeptide architectures with diverse side-chain populations. ACS Nano 2017, 11, 2858–2871.
Hou, Y.; Zhou, Y.; Wang, H.; Wang, R.; Yuan, J.; Hu, Y.; Sheng, K.; Feng, J.; Yang, S.; Lu, H. Macrocyclization of interferonpoly(alpha-amino acid) conjugates significantly improves the tumor retention, penetration, and antitumor efficacy. J. Am. Chem. Soc. 2018, 140, 1170–1178.
Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93.
He, H.; Zheng, N.; Song, Z. Y.; Kim, K. H.; Yao, C.; Zhang, R. J.; Zhang, C. L.; Huang, Y. H.; Uckun, F. M.; Cheng, J. J.; Zhang, Y. F.; Yin, L. C. Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano 2016, 10, 1859–1870.
Mowery, B. P.; Lee, S. E.; Kissounko, D. A.; Epand, R. F.; Epand, R. M.; Weisblum, B.; Stahl, S. S.; Gellman, S. H. Mimicry of antimicrobial host-defense peptides by random copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476.
Zhou, C.; Qi, X.; Li, P.; Chen, W. N.; Mouad, L.; Chang, M. W.; Leong, S. S.; Chan-Park, M. B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of α-aminoacid-N-cyrnhxyanhydrides. Biomacromolecules 2010, 11, 60–67.
Tang, H.; Zhang, D. General route toward side-chain-functionalized α-helical polypeptides. Biomacromolecules 2010, 11, 1585–1592.
Li, P.; Zhou, C.; Rayatpisheh, S.; Ye, K.; Poon, Y. F.; Hammond, P. T.; Duan, H.; Chan-Park, M. B. Cationic peptidopolysaccharides show excellent broad-spectrum antimicrobial activities and high selectivity. Adv. Mater. 2012, 24, 4130–4137.
Ong, Z. Y.; Wiradharma, N.; Yang, Y. Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014, 78, 28–45.
Wang, M.; Zhou, C.; Chen, J.; Xiao, Y.; Du, J. Multifunctional biocompatible and biodegradable folic acid conjugated poly(ε-caprolactone)-polypeptide copolymer vesicles with excellent antibacterial activities. Bioconjug. Chem. 2015, 26, 725–734.
Xiong, M.; Han, Z.; Song, Z.; Yu, J.; Ying, H.; Yin, L.; Cheng, J. Bacteria-assisted activation of antimicrobial polypeptides by a random-coil to helix transition. Angew. Chem. Int. Ed. 2017, 56, 10826–10829.
Teng, P.; Ma, N.; Cerrato, D. C.; She, F.; Odom, T.; Wang, X.; Ming, L. J.; van der Vaart, A.; Wojtas, L.; Xu, H.; Cai, J. Right-handed helical foldamers consisting of de novo D-AApeptides. J. Am. Chem. Soc. 2017, 139, 7363–7369.
Gao, Y. F.; Dong, C. M. Triple redox/temperature responsive diselenide-containing homopolypeptide micelles and supramolecular hydrogels thereof. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 1067–1077.
Zhang, S.; Xiao, X. M.; Qi, F.; Ma, P. C.; Zhang, W. W.; Dai, C. Z.; Zhang, D. F.; Liu, R. H. Biofilm disruption utilizing alpha/beta chimeric polypeptide molecular brushes. Chinese J. Polym. Sci. 2019, 37, 1105–1112.
Huang, J. G.; Li, J. C.; Lyu, Y.; Miao, Q. Q.; Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 2019, 18, 1133–1143.
Xu, D. Z.; Liu, M. Y.; Huang, Q.; Chen, J. Y.; Huang, H. Y.; Deng, F. J.; Tian, J. W.; Wen, Y. Q.; Zhang, X. Y.; Wei, Y. A novel method for the preparation of fluorescent C60 poly(amino acid) composites and their biological imaging. J. Colloid Interf. Sci. 2018, 516, 392–397.
Wu, L.; Zou, Y.; Deng, C.; Cheng, R.; Meng, F.; Zhong, Z. Intracellular release of doxorubicin from core-crosslinked polypeptide micelles triggered by both pH and reduction conditions. Biomaterials 2013, 34, 5262–5272.
Checco, J. W.; Kreitler, D. F.; Thomas, N. C.; Belair, D. G.; Rettko, N. J.; Murphy, W. L.; Forest, K. T.; Gellman, S. H. Targeting diverse protein-protein interaction interfaces with α/β-peptides derived from the Z-domain scaffold. Proc. Natl. Acad. Sci. 2015, 112, 4552–4557.
Han, S.; Li, Z.; Zhu, J.; Han, K.; Zeng, Z.; Hong, W.; Li, W.; Jia, H.; Liu, Y.; Zhuo, R.; Zhang, X. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554.
Kirberger, S. E.; Maltseva, S. D.; Manulik, J. C.; Einstein, S. A.; Weegman, B. P.; Garwood, M.; Pomerantz, W. C. K. Synthesis of intrinsically disordered fluorinated peptides for modular design of high-signal (19) F MRI Agents. Angew. Chem. Int. Ed. 2017, 56, 6440–6444.
Zhou, X.; Li, Z. Advances and biomedical applications of polypeptide hydrogels derived from α-amino acid N-carboxyanhydride (NCA) polymerizations. Adv. Healthc. Mater. 2018, 7, 1800020.
Li, B.; Wu, Y. M.; Zhang, W. J.; Zhang, S.; Shao, N.; Zhang, W. W.; Zhang, L. X.; Fei, J.; Dai, Y. D.; Liu, R. H. Efficient synthesis of amino acid polymers for protein stabilization. Biomater. Sci. 2019, 7, 3675–3682.
Grazon, C.; Salas-Ambrosio, P.; Ibarboure, E.; Buol, A.; Garanger, E.; Grinstaff, M. W.; Lecommandoux, S.; Bonduelle, C. Aqueous ring-opening polymerization-induced self-assembly (ROPISA) of N-carboxyanhydrides. Angew. Chem. Int. Ed. 2020, 59, 622–626.
Duan, Y.; Zheng, H.; Li, Z.; Yao, Y.; Ding, J.; Wang, X.; Nakkala, J. R.; Zhang, D.; Wang, Z.; Zuo, X.; Zheng, X.; Ling, J.; Gao, C. Unsaturated polyurethane films grafted with enantiomeric polylysine promotes macrophage polarization to a M2 phenotype through PI3K/Akt1/mTOR axis. Biomaterials 2020, 246, 120012.
Leuchs, H. Über die Glycin-carbonsaure. Ber. Dtsch. Chem. Ges. 1906, 39, 857–861.
Lai, H. W.; Chen, X. Y.; Lu, Q.; Bian, Z.; Tao, Y. H.; Wang, X. H. A new strategy to synthesize bottlebrushes with a helical polyglutamate backbone via N-carboxyanhydride polymerization and RAFT. Chem. Commun. 2014, 50, 14183–14186.
Wibowo, S. H.; Sulistio, A.; Wong, E. H.; Blencowe, A.; Qiao, G. G. Polypeptide films via N-carboxyanhydride ring-opening polymerization (NCA-ROP): past, present and future. Chem. Commun. 2014, 50, 4971–4988.
Shen, Y.; Li, Z. B.; Klok, H. A. Polypeptide brushes grown via surface-initiated ring-opening polymerization of α-amino acid N-carboxyanhydrides. Chinese J. Polym. Sci. 2015, 33, 931–946.
Song, Z.; Fu, H.; Wang, J.; Hui, J.; Xue, T.; Pacheco, L. A.; Yan, H.; Baumgartner, R.; Wang, Z.; Xia, Y.; Wang, X.; Yin, L.; Chen, C.; Rodriguez-Lopez, J.; Ferguson, A. L.; Lin, Y.; Cheng, J. Synthesis of polypeptides via bioinspired polymerization of in situ purified N-carboxyanhydrides. Proc. Natl. Acad. Sci. 2019, 116, 10658–10663.
Lv, S.; Kim, H.; Feng, L.; Song, Z.; Yang, Y.; Baumgartner, R.; Tseng, K. Y.; Dillon, S. J.; Leal, C.; Yin, L.; Cheng, J. Unimolecular polypeptide micelles via ultra-fast polymerization of N-carboxyanhydrides. J. Am. Chem. Soc. 2020, DOI: https://doi.org/10.1021/jacs.1020c01173.
Deming, T. J. Facile synthesis of block copolypeptides of defined architecture. Nature 1997, 390, 386–389.
Deming, T. J. Transition metal-amine initiators for preparation of well-defined poly(gamma-benzyl L-glutamate). J. Am. Chem. Soc. 1997, 119, 2759–2760.
Zhao, W.; Lv, Y.; Li, J.; Feng, Z.; Ni, Y.; Hadjichristidis, N. Fast and selective organocatalytic ring-opening polymerization by fluorinated alcohol without a cocatalyst. Nat. Commun. 2019, 10, 3590.
Peng, H.; Ling, J.; Shen, Z. Q. Ring opening polymerization of α-amino acid N-carboxyanhydrides catalyzed by rare earth catalysts: polymerization characteristics and mechanism. J. Polym. Sci., Part A: Polym. Chem. 2012, 50, 1076–1085.
Peng, H.; Chen, W. L.; Kong, J.; Shen, Z. Q.; Ling, J. Synthesis of alpha-hydroxy-omega-aminotelechelic polypeptide from alpha-amino acid N-carboxyanhydrides catalyzed by alkali-metal borohydrides. Chinese J. Polym. Sci. 2014, 32, 743–750.
Lu, H.; Cheng, J. Hexamethyldisilazane-mediated controlled polymerization of alpha-amino acid N-carboxyanhydrides. J. Am. Chem. Soc. 2007, 129, 14114–14115.
Lu, H.; Cheng, J. N-trimethylsilyl amines for controlled ring-opening polymerization of amino acid N-carboxyanhydrides and facile end group functionalization of polypeptides. J. Am. Chem. Soc. 2008, 130, 12562–12563.
Yuan, J.; Sun, Y.; Wang, J.; Lu, H. Phenyl trimethylsilyl sulfidemediated controlled ring-opening polymerization of α-amino acid N-carboxyanhydrides. Biomacromolecules 2016, 17, 891–896.
Dimitrov, I.; Schlaad, H. Synthesis of nearly monodisperse polystyrene-polypeptide block copolymers via polymerisation of N-carboxyanhydrides. Chem. Commun. 2003, 2944–2945.
Conejos-Sanchez, I.; Duro-Castano, A.; Birke, A.; Barz, M.; Vicent, M. J. A controlled and versatile NCA polymerization method for the synthesis of polypeptides. J. Polym. Sci., Part A: Polym. Chem. 2013, 4, 3182–3186.
Vacogne, C. D.; Schlaad, H. Primary ammonium/tertiary amine-mediated controlled ring opening polymerisation of amino acid N-carboxyanhydrides. Chem. Commun. 2015, 51, 15645–15648.
Bhaw-Luximon, A.; Jhurry, D.; Belleney, J.; Goury, V. Polymerization of γ-methylglutamate N-carboxyanhydride using Al-Schiff’s base complexes as initiators. Macromolecules 2003, 36, 977–982.
Zhang, H. Y.; Nie, Y. Z.; Zhi, X. M.; Du, H. F.; Yang, J. Controlled ring-opening polymerization of α-amino acid N-carboxyanhydride by frustrated amine/borane Lewis pairs. Chem. Commun. 2017, 53, 5155–5158.
Yuan, J. S.; Zhang, Y.; Li, Z. Z.; Wang, Y. Y.; Lu, H. A S-Sn Lewis pair-mediated ring-opening polymerization of α-amino acid N-carboxyanhydrides: fast kinetics, high Molecular weight, and facile bioconjugation. ACS Macro Lett. 2018, 7, 892–897.
Zhao, W.; Gnanou, Y.; Hadjichristidis, N. Organocatalysis by hydrogen-bonding: a new approach to controlled/living polymerization of α-amino acid N-carboxyanhydrides. J. Polym. Sci., Part A: Polym. Chem. 2015, 6, 6193–6201.
Zou, J.; Fan, J.; He, X.; Zhang, S.; Wang, H.; Wooley, K. L. A facile glovebox-free strategy to significantly accelerate the syntheses of well-defined polypeptides by N-carboxyanhydride (NCA) ring opening polymerizations. Macromolecules 2013, 46, 4223–4226.
Aliferis, T.; Iatrou, H.; Hadjichristidis, N. Living polypeptides. Biomacromolecules 2004, 5, 1653–1656.
Habraken, G. J. M.; Wilsens, K. H. R. M.; Koning, C. E.; Heise, A. Optimization of N-carboxyanhydride (NCA) polymerization by variation of reaction temperature and pressure. Polym. Chem. 2011, 2, 1322–1330.
Pickel, D. L.; Politakos, N.; Avgeropoulos, A.; Messman, J. M. A mechanistic study of α-(amino acid)-N-carboxyanhydride polymerization: comparing initiation and termination events in high-vacuum and traditional polymerization techniques. Macromolecules 2009, 42, 7781–7788.
Vayaboury, W.; Giani, O.; Cottet, H.; Deratani, A.; Schue, F. Living polymerization of α-amino acid N-carboxyanhydrides (NCA) upon decreasing the reaction temperature. Macromol. Rapid Commun. 2004, 25, 1221–1224.
Habraken, G. J. M.; Peeters, M.; Dietz, C. H. J. T.; Koning, C. E.; Heise, A. How controlled and versatile is N-carboxy anhydride (NCA) polymerization at 0 °C? Effect of temperature on homo-, block- and graft (co)polymerization J. Polym. Sci., Part A: Polym. Chem. 2010, 1, 514–524.
Wu, Y.; Zhang, D.; Ma, P.; Zhou, R.; Hua, L.; Liu, R. Lithium hexamethyldisilazide initiated superfast ring opening polymerization of α-amino acid N-carboxyanhydrides. Nat. Commun. 2018, 9, 5297.
Ding, J. D. Glovebox free and rapid ring-opening polymerization of α-amino acid N-carboxyanhydrides in open-vessels. J. Funct. Polym 2019, 32, 120–122.
Wan, X. H.; Wang, X. H. Catalytic system for superfast polypeptide synthesis under atmosphere condition. Acta Polymerica Sinica (in Chinese) 2019, 50, 99–101.
Shifang, L. Facile preparation of polypeptides: moisture insensitive and superfast ring opening polymerization of N-carboxyanhydri. Mater. Rep. 2019, 33, 1–2.
Huesmann, D.; Birke, A.; Klinker, K.; Turk, S.; Rader, H. J.; Barz, M. Revisiting secondary structures in NCA polymerization: influences on the analysis of protected polylysines. Macromolecules 2014, 47, 928–936.
Neufeld, R.; Michel, R.; Herbst-Irmer, R.; Schone, R.; Stalke, D. Introducing a hydrogen-bond donor into a weakly nucleophilic bronsted base: alkali metal hexamethyldisilazides (MHMDS, M = Li, Na, K, Rb and Cs) with ammonia. Chem. Eur. J. 2016, 22, 12340–12346.
Kricheldorf, H. R. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. 2006, 45, 5752–5784.
Kuo, S. W.; Tsai, H. T. Control of peptide secondary structure on star shape polypeptides tethered to polyhedral oligomeric silsesquioxane nanoparticle through click chemistry. Polymer 2010, 51, 5695–5704.
Yu, S. L.; Xiang, X. H.; Zholu, J. L.; Qiu, T.; Hu, Z. X.; Zhu, M. F. Typical polymer fiber materials: an overview and outlook. Acta Polymerica Sinica (in Chinese) 2020, 51, 39–54.
Guo, L.; Lahasky, S. H.; Ghale, K.; Zhang, D. H. N-heterocyclic carbene-mediated zwitterionic polymerization of N-substituted N-carboxyanhydrides toward poly(α-peptoid)s: kinetic, mechanism, and architectural control. J. Am. Chem. Soc. 2012, 134, 9163–9171.
Kricheldorf, H. R.; von Lossow, C.; Schwarz, G. Cyclic polypeptides by solvent-induced polymerizations of α-amino acid N-carboxyanhydrides. Macromolecules 2005, 38, 5513–5518.
Schmid, R. Re-interpretation of the solvent dielectric constant in coordination chemical terms. J. Sol. Chem. 1983, 12, 135–152.
Deng, C.; Wu, J.; Cheng, R.; Meng, F.; Klok, H.; Zhong, Z. Functional polypeptide and hybrid materials: Precision synthesis via α-amino acid N-carboxyanhydride polymerization and emerging biomedical applications. Prog. Polym. Sci. 2014, 39, 330–364.
Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; Shao, N.; Liu, L.; Wu, Y.; Liu, R. Poly(2-ocazoline)-based functional peptide mimics: eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419.
Idelson, M.; Blout, E. High molecular weight poly-α,L-glutamic acid: preparation and optical rotation changes. J. Am. Chem. Soc. 1958, 80, 4631–4634.
Seeney, C. E.; Harwood, H. J. Carbamate ions as propagating species in N-carboxy anhydride polymerizations. J. Macromol. Sci. A Chem. 1975, 9, 779–795.
Kricheldorf, H. R.; von Lossow, C.; Schwarz, G. Tertiary amine catalyzed polymerizations of α-amino acid N-carboxyanhydrides: the role of cyclization. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 4680–4695.
Jiang, W. N.; Xiao, X. M.; Wu, Y. M.; Zhang, W. W.; Cong, Z. H.; Liu, J. J.; Chen, S.; Zhang, H. D.; Xie, J. Y.; Deng, S.; Chen, M. Z.; Wang, Y.; Shao, X. Y.; Dai, Y. D.; Sun, Y.; Fei, J.; Liu, R. H. Peptide polymer displaying potent activity against clinically isolated multidrug resistant Pseudomonas aeruginosa in vitro and in vivo. Biomater. Sci. 2020, 8, 739–745.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21774031 and 21861162010), the National Key Research and Development Program of China (No. 2016YFC1100401), Research Program of State Key Laboratory of Bioreactor Engineering, State Key Laboratory for Modification of Chemical Fibers and Polymer Materials Donghua University, the Fundamental Research Funds for the Central Universities (Nos. 22221818014 and 50321041917001). The authors also thank Research Center of Analysis and Test of East China University of Science and Technology for the help on the characterization.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Information
10118_2020_2471_MOESM1_ESM.pdf
Facile Synthesis of High Molecular Weight Polypeptides via Fast and Moisture Insensitive Polymerization of α-Amino Acid N-Carboxyanhydrides
Rights and permissions
About this article
Cite this article
Wu, YM., Zhang, WW., Zhou, RY. et al. Facile Synthesis of High Molecular Weight Polypeptides via Fast and Moisture Insensitive Polymerization of α-Amino Acid N-Carboxyanhydrides. Chin J Polym Sci 38, 1131–1140 (2020). https://doi.org/10.1007/s10118-020-2471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-020-2471-1