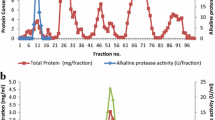
Abstract
The temperature and pH stability of proteases have been extensively investigated. A neutral protease produced by Aspergillus oryzae was thermodynamically characterized at high-salt environment. When the protease was tested at 24% NaCl and 60°C, its half-life was increased to 30.0 min, 111% longer than that of control. Its Gibbs free energy and activation energy for denaturation in high NaCl concentration solutions were higher than in low salt solutions and increased by 2.1 and 4.75 kJ/mol, respectively. The protease exhibited higher thermal stability in higher salt conditions. This feature is beneficial to soybean sauce fermentation by enhancing the protease performance and taste of the product. Analysis by farultraviolet circular dichroism (far-UV CD) spectroscopy revealed that α-helix conformation in the protease increased from 3.2 to 31.7%, respectively, when the NaCl concentration increased from 0 to 18%, in agreement with the results deduced by thermodynamic calculations.
Similar content being viewed by others
References
Ikasari L, Mitchell DA. Leaching and characterization of Rhizopus oligosporus acid protease from solid-state fermentation. Enzyme Microb. Tech. 19: 171–175 (1996)
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 62: 597–635 (1998)
Luh B. Industrial production of soy sauce. J. Ind. Microbiol. Biot. 14: 467–471 (1995)
Impoolsup A, Bhumiratana A, Flegel TW. Isolation of alkaline and neutral proteases from Aspergillus flavus var. columnaris, a soy sauce koji mold. Appl. Environ. Microbiol. 42: 619–628 (1981)
Makhatadze GI, Privalov PL. Energetics of protein sturcture. Adv. Protein Chem. 47: 307–425 (1995)
Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys. J. 71: 2056–2063 (1996)
Matsuo K, Watanabe H, Tate S, Tachibana H, Gekko K. Comprehensive secondary-structure analysis of disulfide variants of lysozyme by synchrotron-radiation vacuum-ultraviolet circular dichroism. Proteins 77: 191–201 (2009)
Wang D, Zheng ZY, Feng J, Zhan XB, Zhang LM, Wu JR, Lin CC, Zhu L. A high-salt-tolerant neutral protease from Aspergillus oryzae: Purification, characterization and kinetic properties. Appl. Biochem. Microbiol. 49: 378–385 (2013)
Devaraj K, Gowda LR, Prakash V. An unusual thermostable aspartic protease from the latex of Ficus racemosa (L.). Phytochem. 69: 647–655 (2008)
Hernández-Martínez R, Gutiérrez-Sánchez G, Bergmann CW, Loera-Corral O, Rojo-Domínguez A, Huerta-Ochoa S, Regalado-González C, Prado-Barragán LA. Purification and characterization of a thermodynamic stable serine protease from Aspergillus fumigatus. Process Biochem. 46: 2001–2006 (2011)
Kristjansson M, Kinsella J. Protein and enzyme stability: Structural, thermodynamic, and experimental aspects. Adv. Food Nutr. Res. 35: 237–316 (1991)
Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 22: 79–89 (1938)
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275 (1951)
Dogan N, Tari C. Characterization of three-phase partitioned exopolygalacturonase from Aspergillus sojae with unique properties. Biochem. Eng. J. 39: 43–50 (2008)
Ortega N, de Diego S, Perez-Mateos M, Busto M. Kinetic properties and thermal behaviour of polygalacturonase used in fruit juice clarification. Food Chem. 88: 209–217 (2004)
Siddiqui K, Rashid M, Rajoka M. Kinetic analysis of the active site of an intracellular β-glucosidase from Cellulomonas biazotea. Folia Microbiol. 42: 53–58 (1997)
Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 287: 252–260 (2000)
Iqbal Z, Rashid M, Jabbar A, Malana M, Khalid A, Rajoka M. Kinetics of enhanced thermostability of an extracellular glucoamylase from Arachniotus sp. Biotech. Lett. 25: 1667–1670 (2003)
Olusesan AT, Azura LK, Forghani B, Bakar FA, Mohamed AKS, Radu S, Manap MYA, Saari N. Purification, characterization and thermal inactivation kinetics of a non-regioselective thermostable lipase from a genotypically identified extremophilic Bacillus subtilis NS 8. New Biotech. 28: 738–745 (2011)
Segel IH. Enzyme Kinetics. Biochemical Calculation. 2nd ed. John Wiley and Sons, New York, NY, USA. p. 197. (1976)
Pace CN, Grimsley GR. Ribonuclease T1 is stabilized by cation and anion binding. Biochem. 27: 3242–3246 (1988)
Nishimura C, Uversky VN, Fink AL. Effect of salts on the stability and folding of staphylococcal nuclease. Biochem. 40: 2113–2128 (2001)
Wright DB, Banks DD, Lohman JR, Hilsenbeck JL, Gloss LM. The effect of salts on the activity and stability of Escherichia coli and Haloferax volcanii dihydrofolate reductases. J. Mol. Biol. 323: 327–344 (2002)
Gloss LM, Topping TB, Binder AK, Lohman JR. Kinetic folding of Haloferax volcanii and Escherichia coli dihydrofolate reductases: Haloadaptation by unfolded state destabilization at high ionic strength. J. Mol. Biol. 376: 1451–1462 (2008)
Toniolo C, Bonora G, Salardi S, Mutter M. Linear oligopeptides. 57. A circular dichroism study of α-helix and β-structure formation in solution by homooligo-L-methionines. Macromolecules 12: 620–625 (1979)
Combes D, Ye W, Zwick A, Monsan P. Effect of salts on enzyme stability. Ann. NY Acad. Sci. 542: 7–10 (1988)
Karan R, Khare S. Stability of haloalkaliphilic Geomicrobium sp. protease modulated by salt. Biochemistry-Moscow+ 76: 686–693 (2011)
Rao L, Zhao X, Pan F, Li Y, Xue Y, Ma Y, Lu JR. Solution behavior and activity of a halophilic esterase under high salt concentration. PloS one 4: e6980 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Zheng, ZY., Feng, J. et al. Influence of sodium chloride on thermal denaturation of a high-salt-tolerant neutral protease from Aspergillus oryzae . Food Sci Biotechnol 22, 1–7 (2013). https://doi.org/10.1007/s10068-013-0223-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-013-0223-5