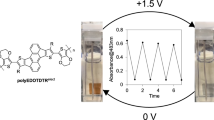
Abstract
Two novel furan and pyridinechalcogenodiazole based monomers, namely 4,7-di(furan-2-yl)-[1, 2, 5]thiadiazolo[3,4-c]pyridine (Fu-S-Fu) and 4,7-di(furan-2-yl)-[1, 2, 5]selenadiazolo [3,4-c]pyridine (Fu-Se-Fu), where a single atom in the pyridinechalcogenodiazole unit is varied from S to Se, were designed and synthesized via a donor-acceptor approach, and then the corresponding polymers, P(Fu-S-Fu) and P(Fu-Se-Fu), were electrosynthesized. Also, structure characterization and optoelectronic properties, including FTIR, SEM, DFT theoretical calculations, intramolecular charge transfer nature, optical and electrochemical behaviors, and electrochromic performance, were systematically investigated and comparatively discussed. The obtained monomers exhibited lower oxidation potential (Fu-S-Fu: 1.12 V; Fu-Se-Fu: 1.09 V), leading to the facile electrodeposition of uniform hybrid polymer films with outstanding electroactivity at low oxidation potentials. Optical spectroscopy of corresponding polymers showed that Se substitution led to a red-shift in the low-energy transition, while the high-energy band remains relatively constant in energy.












Similar content being viewed by others
References
Skotheim TA, Reynolds JR (2007) Handbook of conducting polymers, 3rd edn. CRC Press, Taylor & Francis Group, Boca Raton
Sadki S, Schottland P, Brodie N, Sabouraud G (2000) The mechanisms of pyrrole electropolymerization. Chem Soc Rev 29:283–293
Perepichka IF, Perepichka DF (2009) Handbook of thiophene-based materials: applications in organic electronics and photonics. Wiley, Chichester, West Sussex, England
Patra A, Wijsboom YH, Zade SS, Li M, Sheynin Y, Leitus G, Bendikov M (2008) Poly(3,4-ethylenedioxyselenophene). J Am Chem Soc 130:6734–6736
Patra A, Bendikov M (2010) Polyselenophenes. J Mater Chem 20:422–433
Gidron O, Diskin-Posner Y, Bendikov M (2010) α-oligofurans. J Am Chem Soc 132:2148–2150
Walker B, Tomayo AB, Dang XD, Zalar P, Seo JH, Garcia A, Tantiwi-wat M, Nguyen TQ (2009) Nanoscale phase separation and high photovoltaic efficiency in solution-processed, small-molecule bulk heterojunction solar cells. Adv Funct Mater 19:3063–3069
İҫli-Ӧzkut M, İpek H, Karabay B, Cihaner A, Ӧnal AM (2013) Furan and benzochalcogenodiazole based multichromic polymers via a donor-acceptor approach. Polym Chem 4:2457–2463
Lin JT, Chen PC, Yen YS, Hsu YC, Chou HH, Yeh MCP (2008) Organic dyes containing furan moiety for high-performance dye-sensitized solar cells. Org Lett 11:97–100
Gidron O, Dadvand A, Sheynin Y, Bendikov M, Perepichka DF (2011) Towards “green” electronic materials. α-oligofurans as semiconductors. Chem Commun 47:1976–1978
McConnell RM, Godwin WE, Baker SE, Powell K, Baskett M, Morara A (2004) Polyfuran and co-polymers: a chemical synthesis. Int J Polym Mater 53:697–708
Distefano G, Jones D, Guerra M, Favaretto L, Modelli A, Mengoli G (1991) Determination of the electronic structure of oligofurans and extrapolation to polyfuran. J Phys Chem 95:9746–9753
Demirboğa B, Ӧnal AM (1999) Electrochemical polymerization of furan and 2-methylfuran. Synth Met 99:237–242
Tirkesx S, Ӧnal AM (2007) Electrosynthesis of polyfuran in acetonitrile–boron trifluoride–ethyl ether mixture and its device application. J Appl Polym Sci 103:871–876
Tourillon G, Garnier F (1982) New electrochemically generated organic conducting polymers. J Electroanal Chem 135:173–178
Ohsawa T, Kaneto K, Yoshino K (1984) Electrical and optical properties of electrochemically prepared polyfuran. Jpn J Appl Phys 23:L663
Glenis S, Benz M, LeGoff E, Schindler JL, Kannewurf CR, Kanatzidis MG (1993) Polyfuran:a new synthetic approach and electronic properties. J Am Chem Soc 115:12519–12525
Wan XB, Yan F, Jin S, Liu XR, Xue G (1999) Low potential electrochemical synthesis of polyfuran and characterization of the obtained free-standing film. Chem Mater 11:2400–2407
Peart PA, Tovar JD (2009) Expanding the realm of furan-based conducting polymers through conjugation with 1, 6-methano[10]annulene. Macromolecules 42:4449–4455
Bijleveld JC, Karsten BP, Mathijssen SGJ, Wienk MM, de Leeuw DM, Janssen RAJ (2011) Small band gap copolymers based on furan and diketopyrrolopyrrole for field-effect transistors and photovoltaic cells. J Mater Chem 21:1600–1606
Yiu AT, Beaujuge PM, Lee OP, Woo CH, Toney MF, JMJ F (2012) Side-chain tunability of furan-containing low-band-gap polymers provides control of structural order in efficient solar cells. J Am Chem Soc 134:2180–2185
Sonar P, Foong TRB, Singh SP, Li Y, Dodabalapur A (2012) A furan-containing conjugated polymer for high mobility ambipolar organic thin film transistors. Chem Commun 48:8383–8385
Cheng YJ, Yang SH, Hsu CS (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109:5868–5923
Beaujuge PM, Reynolds JR (2010) Color control in π-conjugated organic polymers for use in electrochromic devices. Chem Rev 110:268–320
Beaujuge PM, Amb CM, Reynolds JR (2010) Spectral engineering in π-conjugated polymers with intramolecular donor-acceptor interactions. Acc Chem Res 43:13961407
Zhang L, Colella NS, Liu F, Trahan S, Baral JK, Winter HH, Mannsfeld SCB, Briseno AL (2013) Synthesis, electronic structure, molecular packing/morphology evolution, and carrier mobilities of pure oligo−/poly (alkylthiophenes). J Am Chem Soc 135:844–854
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131:1979–1985
Blouin N, Michaud A, Gendron D, Wakim S, Blair E, Neagu-Plesu R, Belletête M, Durocher G, Tao Y, Leclerc M (2008) Toward a rational design of poly (2,7-carbazole) derivatives for solar cells. J Am Chem Soc 130:732–742
Liu J, Chen Q (2010) Advances in synthesis and application of imidazopyridine derivatives. Prog Chem 22:631–638
Sun Y, Chien SC, Yip HL, Zhang Y, Chen KS, Zeigler DF, Chen FC, Lin B, Jen AKY (2011) High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3,4-c] pyridine units for thin film transistor and photovoltaic applications. J Mater Chem 21:13247–13255
Zhou H, Yang L, Price SC, Knight KJ, You W (2010) Enhanced photovoltaic performance of low-bandgap polymers with deep LUMO levels. Angew Chem 122:8164–8167
Zhen SJ, Lu BY, Xu JK, Zhang SM, Li YZ (2014) Poly(mono-, bi- or trifuran): effect of oligomer chain length on the electropolymerization performances and polymer properties. RSC Adv 4:14001–14012
Zhen SJ, Xu JK, Lu BY, Zhang SM, Zhao L, Li J (2014) Tuning the optoelectronic properties of polyfuran by design of furan-EDOT monomers and free-standing films with enhanced redox stability and electrochromic performances. Electrochim Acta 146:666–678
Ming SL, Zhen SJ, Lin KW, Zhao L, Xu JK, Lu BY (2015) Thiadiazolo[3,4-c]pyridine as an acceptor toward fast-switching green donor-acceptor-type electrochromic polymer with low bandgap. ACS Appl Mater Interfaces 7:11089–11098
Pommerehne J, Vestweber H, Guss W, Mahrt RF, Bässler H, Porsch M, Daub J (1995) Efficient two layer leds on a polymer blend basis. Adv Mater 7:551–554
Zhu SS, Swager TM (1997) Conducting polymetallorotaxanes: metal ion mediated enhancements in conductivity and charge localization. J Am Chem Soc 119:12568–12577
Cihaner A, Algi F (2008) A novel neutral state green polymeric electrochromic with superior n- and p-doping processes: closer to red-blue-green (RGB) display realization. Adv Funct Mater 18:3583–3589
İҫli M, Pamuk M, Algi F, Ӧnal AM, Cihaner A (2010) Donor-acceptor polymer electrochromes with tunable colors and performance. Chem Mater 22:4034–4044
Dong B, Xing YH, Xu JK, Zheng LQ, Hou J, Zhao F (2008) Electrosyntheses of free-standing and highly conducting polyselenophene films in an ionic liquid. Electrochim Acta 53:5745–5751
Greory LG, Theresa MM, Dwight SS (2012) Atomistic band gap engineering in donor–acceptor polymers. J Am Chem Soc 134:539–547
Kulkarni AP, Zhu Y, Babel A, Wu PT, Jenekhe SA (2008) New ambipolar organic semiconductors. 2. Effects of electron acceptor strength on intramolecular charge transfer photophysics, highly efficient electroluminescence, and field-effect charge transport of phenoxazine-based donor-acceptor materials. Chem Mater 20:4212–4223
Jenekhe SA, Lu L, Alam MM (2001) New conjugated polymers with donor-acceptor architectures: synthesis and photophysics of carbazole-quinoline and phenothiazine-quinoline copolymers and oligomers exhibiting large intramolecular charge transfer. Macromolecules 34:7315–7324
Jordan RB (1999) Reaction mechanisms of inorganic and organometallic systems, ch. 3, 3rd edn. Oxford University Press, USA, p. p. 66
Inzelt G, Pineri M, Schultze JW, Vorotyntsev MA (2000) Electron and proton conducting polymers: recent developments and prospects. Electrochim Acta 45:2403–2421
Baran D, Oktem G, Celebi S, Toppare L (2011) Neutral-state green conjugated polymers from pyrrole bis-substituted benzothiadiazole and benzoselenadiazole for electrochromic devices. Macromol Chem Phys 212:799–805
Acknowledgments
We are grateful to the National Natural Science Foundation of China (grant numbers: 51572117, 51463008, 51303073), Ganpo Outstanding Talents 555 projects (2013), Key Project of Jiangxi Educational Committee (GJJ150795), Scientific Fund of Jiangxi Science & Technology Normal University (2014QNBJRC003), and National Undergraduate Scientific Research Project of China (201511318014) for their financial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hongtao Liu and Shijie Zhen contributed equally to this work.
Electronic supplementary material
ESM 1
(DOC 609 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Zhen, S., Ming, S. et al. Furan and pyridinechalcogenodiazole-based π-conjugated systems via a donor-acceptor approach. J Solid State Electrochem 20, 2337–2349 (2016). https://doi.org/10.1007/s10008-016-3253-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3253-0