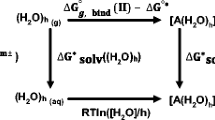
Abstract
The hydration free energies, structures, and dynamics of open- and closed-shell trivalent lanthanide and actinide metal cations are studied using molecular dynamics simulations (MD) based on a polarizable force field. Parameters for the metal cations are derived from an ab initio bottom-up strategy. MD simulations of six cations solvated in bulk water are subsequently performed with the AMOEBA polarizable force field. The calculated first-and second shell hydration numbers, water residence times, and free energies of hydration are consistent with experimental/theoretical values leading to a predictive modeling of f-elements compounds.

Solvation free energy of the actinide (III) and lanthanide (III) cations in water: AMOEBA vs. reference data



Similar content being viewed by others
References
Kaltsoyannis N, Hay PJ, Li J, Blaudeau J-P, Bursten B (2011) Theoretical Studies of the Electronic Structure of Compounds of the Actinide Elements. In: Morss L, Edelstein N, Fuger J (eds) The Chemistry of the Actinide and Transactinide Elements. Springer, Netherlands, pp 1893–2012
Choppin G, Jensen M (2011) Actinides in Solution: Complexation and Kinetics. In: Morss L, Edelstein N, Fuger J (eds) The Chemistry of the Actinide and Transactinide Elements. Springer, Netherlands, pp 2524–2621
David FH (2008) About low oxidation states, hydration and covalence properties of f elements. Radiochim Acta 96(3):135–144
Marcus Y (1994) A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys Chem 51(2–3):111–127
Clavaguéra-Sarrio C, Brenner V, Hoyau S, Marsden CJ, Millié P, Dognon JP (2003) Modeling of uranyl cation − water clusters. J Phys Chem B 107(13):3051–3060
Clavaguera C, Pollet R, Soudan JM, Brenner V, Dognon JP (2005) Molecular dynamics study of the hydration of lanthanum(III) and europium(III) including many-body effects. J Phys Chem B 109(16):7614–7616
Clavaguera C, Calvo F, Dognon JP (2006) Theoretical study of the hydrated Gd3+ ion: structure, dynamics, and charge transfer. J Chem Phys 124(7):74505
Clavaguera C, Sansot E, Calvo F, Dognon JP (2006) Gd(III) polyaminocarboxylate chelate: realistic many-body molecular dynamics simulations for molecular imaging applications. J Phys Chem B 110(26):12848–12851
Jiao D, King C, Grossfield A, Darden TA, Ren P (2006) Simulation of Ca2+ and Mg2+ solvation using polarizable atomic multipole potential. J Phys Chem B 110(37):18553–18559
Piquemal JP, Perera L, Cisneros GA, Ren P, Pedersen LG, Darden TA (2006) Towards accurate solvation dynamics of divalent cations in water using the polarizable amoeba force field: From energetics to structure. J Chem Phys 125(5):054511
Hagberg D, Bednarz E, Edelstein NM, Gagliardi L (2007) A quantum chemical and molecular dynamics study of the coordination of Cm(III) in water. J Am Chem Soc 129(46):14136–14137
Villa A, Hess B, Saint-Martin H (2009) Dynamics and structure of Ln(III)-aqua ions: a comparative molecular dynamics study using ab initio based flexible and polarizable model potentials. J Phys Chem B 113(20):7270–7281
Galbis E, Hernandez-Cobos J, den Auwer C, Le Naour C, Guillaumont D, Simoni E, Pappalardo RR, Sanchez Marcos E (2010) Solving the hydration structure of the heaviest actinide aqua ion known: the californium(III) case. Angew Chem Int Ed Engl 49(22):3811–3815
Wu JC, Piquemal JP, Chaudret R, Reinhardt P, Ren P (2010) Polarizable molecular dynamics simulation of Zn(II) in water using the AMOEBA force field. J Chem Theory Comput 6(7):2059–2070
D’Angelo P, Spezia R (2012) Hydration of lanthanoids(III) and actinoids(III): an experimental/theoretical saga. Chem Eur J 18(36):11162–11178
Real F, Trumm M, Schimmelpfennig B, Masella M, Vallet V (2013) Further insights in the ability of classical nonadditive potentials to model actinide ion-water interactions. J Comput Chem 34(9):707–719
D’Angelo P, Martelli F, Spezia R, Filipponi A, Denecke MA (2013) Hydration properties and ionic radii of actinide(III) ions in aqueous solution. Inorg Chem 52(18):10318–10324
Grossfield A, Ren P, Ponder JW (2003) Ion solvation thermodynamics from simulation with a polarizable force field. J Am Chem Soc 125(50):15671–15682
Semrouni D, Isley Iii WC, Clavaguéra C, Dognon J-P, Cramer CJ, Gagliardi L (2013) Ab initio extension of the AMOEBA polarizable force field to Fe2+. J Chem Theory Comput 9(7):3062–3071
Marjolin A, Gourlaouen C, Clavaguéra C, Ren P, Wu J, Gresh N, Dognon J-P, Piquemal J-P (2012) Toward accurate solvation dynamics of lanthanides and actinides in water using polarizable force fields: from gas-phase energetics to hydration free energies. Theor Chem Acc 131(4):1–14
Gourlaouen C, Clavaguéra C, Marjolin A, Piquemal J-P, Dognon J-P (2013) Understanding the structure and electronic properties of Th4 + −water complexes. Can J Chem 91(9):821–831
Cao X, Dolg M, Stoll H (2003) Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J Chem Phys 118(2):487–496
Dunning JTH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023
Werner HJ, Knowles PJ, Manby FR, Schutz M, Celani P, Knizia G, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Adler TB, Amos RD, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Liu Y, Lloyd AW, Mata RA, May AJ, McNicholas SJ, Meyer W, Mura ME, Nicklass A, Palmieri P, Pflüger K, Pitzer R, Reiher M, Shiozaki T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M, Wolf A (2010) MOLPRO, version 2010.1, a package of ab initio programs
Bagus PS, Hermann K, Bauschlicher JCW (1984) On the nature of the bonding of lone pair ligands to a transition metal. J Chem Phys 81(4):1966–1974
Bauschlicher JCW, Bagus PS, Nelin CJ, Roos BO (1986) The nature of the bonding in XCO for X = Fe, Ni, and Cu. J Chem Phys 85(1):354–364
Piquemal JP, Marquez A, Parisel O, Giessner-Prettre C (2005) A CSOV study of the difference between HF and DFT intermolecular interaction energy values: the importance of the charge transfer contribution. J Comput Chem 26(10):1052–1062
Marjolin A, Gourlaouen C, Clavaguéra C, Dognon JP, Piquemal JP (2013) Towards energy decomposition analysis for open and closed shell f-elements mono aqua complexes. Chem Phys Lett 563:25–29
Clavaguéra C, Dognon JP (2005) Accurate static electric dipole polarizability calculations of +3 charged lanthanide ions. Chem Phys 311(1–2):169–176
Ponder JW (2009) TINKER: software tools for molecular design, version 6.2. 6.2 edn. Washington University School of Medicine, Saint Louis
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Beeman D (1976) Some multistep methods for use in molecular dynamics calculations. J Comput Phys 20(2):130–139
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593
Bennett CH (1976) Efficient estimation of free energy differences from Monte Carlo data. J Comput Phys 22(2):245–268
Allen PG, Bucher JJ, Shuh DK, Edelstein NM, Craig I (2000) Coordination chemistry of trivalent lanthanide and actinide ions in dilute and concentrated chloride solutions. Inorg Chem 39(3):595–601
Penner-Hahn JE (2005) Characterization of “spectroscopically quiet” metals in biology. Coord Chem Rev 249(1–2):161–177
Antonio M, Soderholm L (2011) X-Ray Absorption Spectroscopy of the Actinides. In: Morss L, Edelstein N, Fuger J (eds) The Chemistry of the Actinide and Transactinide Elements. Springer, Netherlands, pp 3086–3198
Bobyr E, Lassila JK, Wiersma-Koch HI, Fenn TD, Lee JJ, Nikolic-Hughes I, Hodgson KO, Rees DC, Hedman B, Herschlag D (2012) High-Resolution Analysis of Zn2+ Coordination in the Alkaline Phosphatase Superfamily by EXAFS and X-ray Crystallography. J Mol Biol 415(1):102–117
Díaz-Moreno S, Ramos S, Bowron DT (2011) Solvation structure and Ion complexation of La3+ in a 1 molal aqueous solution of lanthanum chloride. J Phys Chem A 115(24):6575–6581
Smirnov PR, Trostin VN (2012) Structural parameters of the nearest surrounding of lanthanide ions in aqueous solutions of their salts. Russ J Gen Chem 82(3):360–378
Smirnov PR, Trostin VN (2012) Sructural parameters of the nearest surrounding of tri- and tetravalent actinide ions in aqueous solutions of actinide salts. Russ J Gen Chem 82(7):1204–1213
Impey RW, Madden PA, McDonald IR (1983) Hydration and mobility of ions in solution. J Phys Chem 87(25):5071–5083
Helm L, Merbach AE (2005) Inorganic and bioinorganic solvent exchange mechanisms. Chem Rev 105(6):1923–1959
Skanthakumar S, Antonio MR, Wilson RE, Soderholm L (2007) The curium aqua Ion. Inorg Chem 46(9):3485–3491
D'Angelo P, Zitolo A, Migliorati V, Chillemi G, Duvail M, Vitorge P, Abadie S, Spezia R (2011) Revised ionic radii of lanthanoid(III) ions in aqueous solution. Inorg Chem 50(10):4572–4579
Farkas I, Grenthe I, Bányai I (2000) The rates and mechanisms of water exchange of actinide aqua ions: a variable temperature 17O NMR study of U(H2O)104+, UF(H2O)93+, and Th(H2O)104+. J Phys Chem A 104(6):1201–1206
David FH, Vokhmin V (2001) Hydration and entropy model for ionic and covalent monatomic ions. J Phys Chem A 105(42):9704–9709
Joung IS, Cheatham TE 3rd (2008) Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B 112(30):9020–9041
Acknowledgments
P. Y. R. thanks support by the National Institute of Health (R01GM106137). A.M. thanks the CEA for a PhD grant. J.-P. D. thanks the CEA nuclear energy division DEN/RBPCH for financial support. This work was granted access to the HPC resources of [CCRT/CINES/IDRIS] under the allocation c2013086146 made by GENCI (Grand Equipement National de Calcul Intensif).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper belongs to Topical Collection QUITEL 2013
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
MRCI results; validation on metal-water cluster interaction energies; structural data from SBC 216 H2O and PBC 215 H2O MD; hydration Gibbs free energies. Supporting information for this article is available on the WWW under http://dx.doi.org/. (DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Marjolin, A., Gourlaouen, C., Clavaguéra, C. et al. Hydration gibbs free energies of open and closed shell trivalent lanthanide and actinide cations from polarizable molecular dynamics. J Mol Model 20, 2471 (2014). https://doi.org/10.1007/s00894-014-2471-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2471-6