Abstract
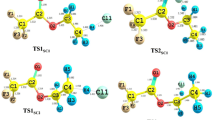
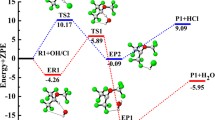
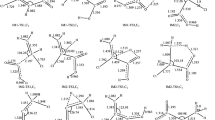
Due to their lack of effect on the ozone depletion, hydrofluoroethers are considered as potential candidates for third generation refrigerants. In the present work, the mechanisms and kinetics of reaction of the Cl atom with CF3CHFOCH3 and CHF2CHFOCF3 were investigated theoretically using quantum chemical methods and transition state theory. Four reaction pathways for the title reaction were explored. By using conventional transition state theory with Eckart tunneling correction, the rate constants of the title reaction were obtained over the temperature range 200–300 K. Kinetic calculations demonstrate that H-abstraction from the –CH3 group in CF3CHFOCH3 and H-abstraction from the –CHF2 group in CHF2CHFOCF3 are major reaction pathways, with the barrier heights of the two paths calculated to be −1.04 and 4.33 kcal mol−1, respectively. However, the contribution of H-abstraction from the –CHFO– group for the two reactions should also be taken into account with increased temperature. At 298 K, the calculated overall rate constants of the reaction of CHF2CHFOCF3 with the Cl atom are 4.27 × 10−15 cm3 molecule−1 s−1, which is consistent with the experimental value of (1.2 ± 2.0) × 10−15 cm3 molecule−1 s−1.



Similar content being viewed by others
References
Seikya A, Misaki S (1997) Proceedings of the International Conference on Ozone Protection Technologies, Baltimore, MD, pp 26
Molina MJ, Rowland FS (1974) Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature 249(5460):810–812
Oyaro N, Sellevåg SR, Nielsen CJ (2004) Atmospheric chemistry of hydrofluoroethers: reaction of a series of hydrofluoroethers with OH radicals and cl atoms, atmospheric lifetimes, and global warming potentials. J Phys Chem A 109(2):337–346. doi:10.1021/jp047860c
Kambanis KG, Lazarou YG, Papagiannakopoulos P (1998) Kinetic study for the reactions of chlorine atoms with a series of hydrofluoroethers. J Phys Chem A 102(44):8620–8625. doi:10.1021/jp982277f
Seikya A, Misaki S (1996) A continuing search for new refrigerants. Chemtech 26 (12)
Sekiya A, Misaki S (2000) The potential of hydrofluoroethers to replace CFCs, HCFCs and PFCs. J Fluorine Chem 101(2):215–221. doi:10.1016/S0022-1139(99)00162-1
Chen L, Kutsuna S, Tokuhashi K, Sekiya A (2006) Kinetics and mechanisms of CF3CHFOCH3, CF3CHFOC(O)H, and FC(O)OCH3 reactions with OH radicals. J Phys Chem A 110(47):12845–12851. doi:10.1021/jp064917h
Imasu R, Suga A, Matsuno T (1995) Radiative effects and halocarbon global warming potentials of replacement compounds for chlorofluorocarbons. J Meteorol Soc Jpn 73(6):1123–1136
Zhang H, Liu Y, Liu J-Y, Li Z-S (2012) Theoretical study and rate constants calculation for the reactions X + CF3CH2OCF3 (X = F, Cl, Br). J Comput Chem 33(6):685–690. doi:10.1002/jcc.22897
Jia X, Liu Y, Sun J, Sun H, Su Z, Pan X, Wang R (2009) Theoretical investigation of the reactions of CF3CHFOCF3 with the OH radical and Cl atom. J Phys Chem A 114(1):417–424. doi:10.1021/jp908228h
Thornton JA, Kercher JP, Riedel TP, Wagner NL, Cozic J, Holloway JS, Dubé WP, Wolfe GM, Quinn PK, Middlebrook AM, Alexander B, Brown SS (2010) A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature 464 (7286):271–274. doi:http://www.nature.com/nature/journal/v464/n7286/suppinfo/nature08905_S1.html
Hammerich AD, Finlayson-Pitts BJ, Gerber RB (2012) NOx reactions on aqueous surfaces with gaseous HCl: formation of a potential precursor to atmospheric Cl atoms. J Phys Chem Lett 3(23):3405–3410. doi:10.1021/jz3014985
Young CJ, Washenfelder RA, Edwards PM, Parrish DD, Gilman JB, Kuster WC, Mielke LH, Osthoff HD, Tsai C, Pikelnaya O, Stutz J, Veres PR, Roberts JM, Griffith S, Dusanter S, Stevens PS, Flynn J, Grossberg N, Lefer B, Holloway JS, Peischl J, Ryerson TB, Atlas EL, Blake DR, Brown SS (2013) Evaluating evidence for Cl sources and oxidation chemistry in a coastal, urban environment. Atmos Chem Phys Discuss 13(5):13685–13720. doi:10.5194/acpd-13-13685-2013
Saiz-Lopez A, von Glasow R (2012) Reactive halogen chemistry in the troposphere. Chem Soc Rev 41(19):6448–6472. doi:10.1039/C2CS35208G
Rudolph J, Koppmann R, Plass-Dülmer C (1996) The budgets of ethane and tetrachloroethene: is there evidence for an impact of reactions with chlorine atoms in the troposphere? Atmos Environ 30(10–11):1887–1894. doi:10.1016/1352-2310(95)00385-1
Sommariva R, von Glasow R (2012) Multiphase halogen chemistry in the tropical Atlantic Ocean. Environ Sci Technol 46(19):10429–10437. doi:10.1021/es300209f
Lawler MJ, Sander R, Carpenter LJ, Lee JD, von Glasow R, Sommariva R, Saltzman ES (2011) HOCl and Cl2 observations in marine air. Atmos Chem Phys 11(15):7617–7628. doi:10.5194/acp-11-7617-2011
Sivaramakrishnan H, Upare AA, Satagopan D, Chambers OR (2011) The preparation of desflurane by the vapor-phase fluorination of isoflurane. Org Process Res Dev 15(3):585–592. doi:10.1021/op100318b
Osterstrom FF, Nielsen OJ, Sulbaek Andersen MP, Wallington TJ (2012) Atmospheric chemistry of CF3CH2OCH3: reaction with chlorine atoms and OH radicals, kinetics, degradation mechanism and global warming potential. Chem Phys Lett 524(0):32–37. doi:10.1016/j.cplett.2011.12.047
Zierkiewicz W (2013) Reaction of volatile anaesthetic desflurane with chlorine atom. Theoretical investigation. Chem Phys Lett 555(0):72–78. doi:10.1016/j.cplett.2012.11.011
Liu H-x, Liu Y-c, Wan S-q, Liu J-y (2010) Reaction of Cl with CF3CH2OCHO: a mechanistic and kinetic study. J Mol Struct (Theochem) 944(1–3):124–131. doi:10.1016/j.theochem.2009.12.041
Beach SD, Hickson KM, Smith IWM, Tuckett RP (2001) Rate constants and Arrhenius parameters for the reactions of OH radicals and Cl atoms with CF3CH2OCHF2, CF3CHClOCHF2 and CF3CH2OCClF2, using the discharge-flow/resonance fluorescence method. Phys Chem Chem Phys 3(15):3064–3069. doi:10.1039/B103883B
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) Kinetic study for the reactions of several hydrofluoroethers with chlorine atoms. J Phys Chem A 108(14):2666–2674. doi:10.1021/jp031081z
Becke AD (1993) Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT
Song G, Jia X, Gao Y, Luo J, Yu Y, Wang R, Pan X (2010) Theoretical studies on the mechanisms and dynamics of OH radicals with CH2FCF2OCHF2 and CH2FOCH2F. J Phys Chem A 114(34):9057–9068. doi:10.1021/jp102421g
Gao H, Wang Y, Wan S-Q, Liu J-Y, Sun C-C (2009) Theoretical investigation of the hydrogen abstraction from CF3CH2CF3 by OH radicals, F, and Cl atoms: a dual-level direct dynamics study. J Mol Struct (THEOCHEM) 913(1–3):107–116. doi:10.1016/j.theochem.2009.07.024
Chan B, Radom L (2011) Assessment of theoretical procedures for hydrogen-atom abstraction by chlorine, and related reactions. Theor Chem Acc 130(2–3):251–260. doi:10.1007/s00214-011-0967-z
Chan B, Radom L (2012) Approaches for obtaining accurate rate constants for hydrogen abstraction by a chlorine atom. J Phys Chem A 116(14):3745–3752. doi:10.1021/jp3007409
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94(14):5523–5527. doi:10.1021/j100377a021
Liu F-Y, Long Z-W, Tan X-F, Long B (2014) Theoretical investigation on mechanisms and kinetics of the reactions of Cl atom with CH3OOH and CH3CH2OOH. Comput Theor Chem 1038(0):33–39. doi:10.1016/j.comptc.2014.04.011
Chow R, Ng M, Mok DKW, Lee EPF, Dyke JM (2014) Rate coefficients of the Cl + CH3C(O)OCH3 → HCl + CH3C(O)OCH2 reaction at different temperatures calculated by transition-state theory with ab initio and density functional theory reaction paths. J Phys Chem A 118(11):2040–2055. doi:10.1021/jp5000864
Brudnik K, Twarda M, Sarzyński D, Jodkowski J (2013) Theoretical study of the kinetics of chlorine atom abstraction from chloromethanes by atomic chlorine. J Mol Model 19(10):4181–4193. doi:10.1007/s00894-013-1779-y
Brudnik K, Twarda M, Sarzyński D, Jodkowski J (2013) Theoretical study of the kinetics of reactions of the monohalogenated methanes with atomic chlorine. J Mol Model 19(4):1489–1505. doi:10.1007/s00894-012-1709-4
Mao W-X, Long Z-W, Long B, Wang Y-B, Long C-Y, Qin S-J (2013) Theoretical study on the gas phase reaction of dimethyl sulfoxide with atomic chlorine in the presence of water. Struct Chem 24(2):383–392. doi:10.1007/s11224-012-0086-z
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M, Celani P, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Shamasundar KR, Adler TB, Amos RD, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Liu Y, Lloyd AW, Mata RA, May AJ, McNicholas SJ, Meyer W, Mura ME, Nicklaß A, O’Neill DP, Palmieri P, Pflüger K, Pitzer R, Reiher M, Shiozaki T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M, Wolf A (2012) MOLPRO version 2012.1. https://www.molpro.net/
Tarnopolsky A, Karton A, Sertchook R, Vuzman D, Martin JML (2007) Double-hybrid functionals for thermochemical kinetics. J Phys Chem A 112(1):3–8. doi:10.1021/jp710179r
Long B, Chang C-R, Long Z-W, Wang Y-B, Tan X-F, Zhang W-J (2013) Nitric acid catalyzed hydrolysis of SO3 in the formation of sulfuric acid: a theoretical study. Chem Phys Lett 581(0):26–29. doi:10.1016/j.cplett.2013.07.012
Alvarez-Idaboy JR, Mora-Diez N, Vivier-Bunge A (2000) A quantum chemical and classical transition state theory explanation of negative activation energies in OH addition to substituted ethenes. J Am Chem Soc 122(15):3715–3720. doi:10.1021/ja993693w
Long B, Tan X-F, Chang C-R, Zhao W-X, Long Z-W, Ren D-S, Zhang W-J (2013) Theoretical studies on gas-phase reactions of sulfuric acid catalyzed hydrolysis of formaldehyde and formaldehyde with sulfuric acid and H2SO4 · · · H2O complex. J Phys Chem A 117(24):5106–5116. doi:10.1021/jp312844z
Gonzalez J, Anglada JM, Buszek RJ, Francisco JS (2011) Impact of water on the OH + HOCl reaction. J Am Chem Soc 133(10):3345–3353. doi:10.1021/ja100976b
Long B, Zhang W-j, Tan X-f, Long Z-w, Wang Y-b, Ren D-s (2011) Theoretical studies on the gas phase reaction mechanisms and kinetics of glyoxal with HO2 with water and without water. Comput Theor Chem 964(1–3):248–256. doi:10.1016/j.comptc.2011.01.003
Long B, Tan X-F, Long Z-W, Wang Y-B, Ren D-S, Zhang W-J (2011) Theoretical studies on reactions of the stabilized H2COO with HO2 and the HO2 · · · H2O complex. J Phys Chem A 115(24):6559–6567. doi:10.1021/jp200729q
Truong TN, Truhlar DG (1990) Ab initio transition state theory calculations of the reaction rate for OH+CH4→H2O+CH3. J Chem Phys 93(3):1761–1769. doi:10.1063/1.459103
Duncan WT, Bell RL, Truong TN (1998) TheRate: program for ab initio direct dynamics calculations of thermal and vibrational-state-selected rate constants. J Comput Chem 19(9):1039–1052. doi:10.1002/(SICI)1096-987X(19980715)19:9<1039::AID-JCC5>3.0.CO;2-R
Yang L, He H-Q, Ji Y-M, Liu J-Y, Li Z-S (2007) Direct ab initio dynamics calculations of the rate constants for the reaction of CHF2CF2OCH3 with Cl. Int J Chem Kinet 39(4):221–230. doi:10.1002/kin.20230
Yang L, Liu J-Y, Wang L, He H-Q, Wang Y, Li Z-S (2008) Theoretical study of the reactions CF3CH2OCHF2 + OH/Cl and its product radicals and parent ether (CH3CH2OCH3) with OH. J Comput Chem 29(4):550–561. doi:10.1002/jcc.20813
Zhang H, C-y L, G-l Z, W-j H, Sun M, Liu B, Z-s L (2010) Theoretical studies of the reactions of Cl atoms with CF3CH2OCHnF(3−n) (n = 1, 2, 3). Theor Chem Acc 127(5–6):551–560. doi:10.1007/s00214-010-0746-2
Lily M, Sutradhar D, Chandra AK (2013) Theoretical investigations on kinetics, mechanism and thermochemistry of the gas phase reactions of CHF2OCF2CHF2 with OH radicals. Comput Theor Chem 1022(0):50–58. doi:10.1016/j.comptc.2013.08.016
Hsu KJ, DeMore WB (1995) Temperature-dependent rate constants and substituent effects for the reactions of hydroxyl radicals with three partially fluorinated ethers. J Phys Chem 99(28):11141–11146. doi:10.1021/j100028a014
Singh H, Rao P, Tiwari L (2013) Theoretical studies on OH and Cl initiated hydrogen atom abstraction of HFE-227pc (CF3OCF2CHF2). J Atmos Chem 70(3):257–268. doi:10.1007/s10874-013-9266-5
Sun H, Gong H, Pan X, Hao L, Sun C, Wang R, Huang X (2009) Theoretical investigation of the reaction of CF3CHFOCH3 with OH radical. J Phys Chem A 113(20):5951–5957. doi:10.1021/jp9006262
Yang L, J-y L, Z-s L (2008) Computational studies on the mechanisms and dynamics of OH reactions with CHF2CHFOCF3 and CHF2CH2OCF3. J Chem Theory Comput 4(7):1073–1082. doi:10.1021/ct800032e
Acknowledgments
This work is supported by National Natural Science Foundation of China (Grant No. 41165007), Science and Technology Foundation of GuiZhou Province, China (No.[2011]2107 and [2012]2189) and Open Research Fund of Key Laboratory of Atmospheric Composition and Optical Radiation, Chinese Academy of Sciences, China (JJ1107).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, FY., Long, ZW., Tan, XF. et al. The reaction mechanisms and kinetics of CF3CHFOCH3 and CHF2CHFOCF3 with atomic chlorine: a computational study. J Mol Model 20, 2435 (2014). https://doi.org/10.1007/s00894-014-2435-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2435-x