Abstract
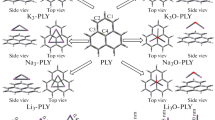
Under high-level ab initio calculations, the geometrical structures and nonlinear optical properties of M@P4 (M=Li, Na, K and Li3O) and M@C3H6 (M=Li and Li3O) were investigated; all were found to exhibit considerable first hyperpolarizabilities (18110, 1440, 22490, 50487, 2757 and 31776 au, respectively). The computational results revealed that when doping the (super)alkali atom M into the tetrahedral P4 molecule, the original dual spherical aromaticity of the P4 moiety is broken and new σ electron cloud is formed on the face of P4 part interacting with the M atom. It was found that interaction of the (super)alkali atom with the σ electron cloud is a novel mode to produce diffuse excess electrons effectively to achieve a considerable β 0 value. Further, beyond the alkali atom, employing the superalkali unit can be a more effective approach to significantly enhance the first hyperpolarizability of the systems, due to the much lower vertical ionization potential. These results were further supported by the case of the (super)alkali atom interacting with the cyclopropane C3H6 molecule with its typical σ aromatic electron cloud. Moreover, the β 0 values of the M@P4 series are nonmonotonic dependent on alkali atomic number, namely, 1440 au (M = Na) < 18110 au (Li) < 22490 au (K), inferring that the distance between the alkali atom and the interacting surface with the σ electron cloud in P4 is a crucial geometrical factor in determining their first hyperpolarizabilities. These intriguing findings will be advantageous for promoting the design of novel high-performance nonlinear optical materials.

A new mode through a (super)alkali atom interacting with the σ electron cloud is proposed to introduce diffuse excess electrons, which leads to large first hyperpolarizability (β 0) in the sampled M@P4 and M@C3H6 (M=Li, Na, K and Li3O) series. Doping the superalkali atom could be an effective approach to enhancing the β 0 value of these systems because of the much lower vertical ionization potential









Similar content being viewed by others
References
Lacroix PG (2001) Second-order optical nonlinearities in coordination chemistry: the case of bis(salicylaldiminato)metal Schiff base complexes. Eur J Inorg Chem 2001:339–348
Marder SR (2006) Organic nonlinear optical materials: where we have been and where we are going. Chem Commun 2:131–134
Zhang C, Song YL, Wang X (2007) Correlations between molecular structures and third-order non-linear optical functions of heterothiometallic clusters: a comparative study. Coordin Chem Rev 251:111–141
Batista RMF, Costa SPG, Belsley M, Lodeiro C, Raposo MMM (2008) Synthesis and characterization of novel (oligo) thienyl-imidazo-phenanthrolines as versatile π-conjugated systems for several optical applications. Tetrahedron 64:9230–9238
Suresh S, Arivuoli D (2012) Nanomaterials for nonlinear optical (NLO) appilcations: a review. Rev Adv Mater Sci 30:243–253
Morley JO, Docherty VJ, Pugh D (1987) Non-linear optical properties of organic molecules. Part 2. Effect of conjugation length and molecular volume on the calculated hyperpolarisabilities of polyphenyls and polyenes. J Chem Soc Perkin Trans II:1351–1355
Blanchard-Desce M, Alain V, Bedworth PV, Marder SR, Fort A, Runser C, Barzoukas M, Lebus S, Wortmann R (1997) Large quadratic hyperpolarizabilities with donor–acceptor polyenes exhibiting optimum bond length alternation: correlation between structure and hyperpolarizability. Chem Eur J 3:1091–1104
Chen W, Yu GT, Gu FL, Aoki Y (2009) Investigation on nonlinear optical properties of ladder-structure polydiacetylenes derivatives by using the elongation finite-field method. Chem Phys Lett 474:175–179
Xiao D, Bulat FA, Yang W, Beratan DN (2008) A donor–nanotube paradigm for nonlinear optical materials. Nano Lett 8:2814–2818
Zhou ZJ, Li XP, Ma F, Liu ZB, Li ZR, Huang XR, Sun CC (2011) Exceptionally large second–order nonlinear optical response in donor–graphene nanoribbon–acceptor systems. Chem Eur J 17:2414–2419
Yu GT, Zhao XG, Niu M, Huang XR, Zhang H, Chen W (2013) Constructing a mixed p-conjugated bridge: a simple and effective approach to realize a large first hyperpolarizability in carbon nanotube-based systems. J Mater Chem C 1:3833–3841
Buey J, Coco S, Díez L, Espinet P, Martín-Alvarez JM, Miguel JA, García-Granda S, Tesouro A, Ledoux I, Zyss J (1998) Synthesis and second-order nonlinear optical properties of new palladium (II) and platinum (II) Schiff-base complexes. Organometallics 17:1750–1755
Labat L, Lamère JF, Sasaki I, Lacroix PG, Vendier L, Asselberghs I, Pérez-Moreno J, Clays K (2006) Synthesis, crystal structure, and second-order nonlinear optical properties of ruthenium(II) complexes with substituted bipyridine and phenylpyridine ligands. Eur J Inorg Chem 2006:3105–3113
Espinet P, Miguel JA, Martín-Alvarez JM, Villacampa B (2010) Synthesis, crystal structure and second-order nonlinear optical properties of the trinuclear palladium orthometalated complex [(μ3-S)(μ3-OH)Pd3(C^N)3] (HC6^N=p-Bun 2N–C6H4–CH=N–C6H4–NO2-p). J Organomet Chem 695:437–440
Bella SD (2001) Second-order nonlinear optical properties of transition metal complexes. Chem Soc Rev 30:355–366
Maury O, Viau L, Senechal K, Corre B, Guegan JP, Renouard T, Ledoux I, Zyss J, Bozec LH (2004) Synthesis, linear and quadratic–nonlinear optical properties of octupolar D3 and D2d bipyridyl metal complexes. Chem Eur J 10:4454–4466
Lee SH, Park JR, Jeong MY, Kim HM, Li SJ, Song J, Ham S, Jeon SJ, Cho BR (2006) First hyperpolarizabilities of 1,3,5-tricyanobenzene derivatives: origin of larger β values for the octupoles than for the dipoles. Chem Phys Chem 7:206–212
Cornelis D, Franz E, Asselberghs I, Clays K, Verbiest T, Koeckelberghs G (2011) Interchromophoric interactions in chiral X-type π-conjugated oligomers: a linear and nonlinear optical study. J Am Chem Soc 133:1317–1327
Chen W, Li ZR, Wu D, Li RY, Sun CC (2005) Theoretical investigation of the large nonlinear optical properties of (HCN)n clusters with Li atom. J Phys Chem B 109:601–608
Muhammad S, Xu HL, Liao Y, Kan Y, Su ZM (2009) Quantum mechanical design and structure of the Li@ B10H14 basket with a remarkably enhanced electro-optical response. J Am Chem Soc 131:11833–11840
Chen W, Li ZR, Wu D, Li Y, Sun CC, Gu FL (2005) The structure and the large nonlinear optical properties of Li@Calix[4]pyrrole. J Am Chem Soc 127:10977–10981
Xu HL, Li ZR, Wu D, Wang BQ, Li Y, Gu FL, Aoki Y (2007) Structures and large NLO responses of new electrides: Li-doped fluorocarbon chain. J Am Chem Soc 129:2967–2970
Yu GT, Huang XR, Chen W, Sun CC (2011) Alkali metal atom-aromatic ring: a novel interaction mode realizes large first hyperpolarizabilities of M@AR (M=Li, Na, and K, AR = pyrrole, indole, thiophene, and benzene). J Comput Chem 32:2005–2011
Hirsch A, Chen ZF, Jiao HJ (2000) Spherical aromaticity in Ih symmetrical fullerenes: the 2(N+1)2 rule. Angew Chem Int Ed 39:3915–3917
Hirsch A, Chen ZF, Jiao HJ (2001) Spherical aromaticity of inorganic cage molecules. Angew Chem Int Ed 40:2834–2838
Chen ZF, Jiao HJ, Hirsch A, Schleyer PR (2002) Spherical homoaromaticity. Angew Chem Int Ed 41:4309–4312
Reiher M, Hirsch A (2003) From rare gas atoms to fullerenes: spherical aromaticity studied from the point of view of atomic structure theory. Chem Eur J 9:5442–5452
Abboud JLM, Alkorta I, Davalos JZ, Gal JF, Herreros M, Maria PC, Mo O, Molina MT, Notario R, Yanez M (2000) The P4⋯Li+ ion in the gas phase: a planetary system. J Am Chem Soc 122:4451–4454
Abboud JLM, Herreros M, Notario R, Esseffar M, MÓ O, Yáňez M (1996) A new bond from an old molecule: formation, stability, and structure of P4H+. J Am Chem Soc 118:1126–1130
Scherer OJ (1990) Complexes with substituent–free acyclic and cyclic phosphorus, arsenic, antimony, and bismuth ligands. Angew Chem Int Ed Engl 29:1104–1122
Krossing I, van Wüllen L (2002) Superweak complexes of tetrahedral P4 molecules with the silver cation of weakly coordinating anions. Chem Eur J 8:700–711
Rehm E, Boldyrev AI, Schleyer PR (1992) Ab initio study of superalkalis. First ionization potentials and thermodynamic stability. Inorg Chem 31:4834–4842
Buckingham AD (1967) The effects of collisions on molecular properties. Adv Chem Phys 12:107–142
Mclean AD, Yoshimine M (1967) Theory of molecular polarizabilities.J. Chem Phys 47:1927–1935
Li Y, Li ZR, Wu D, Li RY, Hao XY, Sun CC (2004) An ab initio prediction of the extraordinary static first hyperpolarizability for the electron-solvated cluster (FH)2{e}(HF). J Phys Chem B 108:3145–3148
Li ZJ, Li ZR, Wang FF, Luo C, Ma F, Wu D, Wang Q, Huang XR (2009) A dependence on the petal number of the static and dynamic first hyperpolarizability for electride molecules: many-petal-shaped Li-doped cyclic polyamines. J Phys Chem A 113:2961–2966
Suponitsky KY, Liao Y, Masunov AE (2009) Electronic hyperpolarizabilities for donor–acceptor molecules with long conjugated bridges: calculations versus experiment. J Phys Chem A 113:10994–11001
Ma F, Li ZR, Xu HL, Li ZJ, Li ZS, Aoki Y, Gu FL (2008) Lithium salt electride with an excess electron pair—a class of nonlinear optical molecules for extraordinary first hyperpolarizability. J Phys Chem A 112:11462–11467
Soscún H, Castellano O, Bermúdez Y, Toro-Mendoza C, Marcano A, Alvarado Y (2002) Linear and nonlinear optical properties of pyridine N-oxide molecule. J Mol Struct THEOCHEM 592:19–28
Sekino H, Maeda Y, Kamiya M, Hirao K (2007) Polarizability and second hyperpolarizability evaluation of long molecules by the density functional theory with long-range correction. J Chem Phys 126:014107
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1996) NBO Version 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, Revision B.01. Gaussian, Inc, Wallingford
Schleyer PR, Maerker C, Dransfeld A, Jiao H, Eikema-Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Kanis DR, Ratner MA, Marks TJ (1994) Design and construction of molecular assemblies with large second-order optical nonlinearities. Quant Chem Asp Chem Rev 94:195–242
Cremer D, Gauss J (1986) Theoretical determination of molecular structure and conformation. 20. Reevaluation of the strain energies of cyclopropane and cyclobutane carbon-carbon and carbon-hydrogen bond energies, 1,3 interactions, and .sigma.-aromaticit. J Am Chem Soc 108:7467–7477
Simon A, Borrmann H, Horakh J (1997) On the polymorphism of white phosphorus. Chem Ber 130:1235–1240
Acknowledgments
This work was supported by National Natural Science Foundation of China (21103065, 21373099, 21073075 and 21173097), National Basic Research Program of China (973 Program) (2012CB932800), and the Ministry of Education of China (20110061120024 and 20100061110046). We acknowledge the High Performance Computing Center (HPCC) of Jilin University for supercomputer time.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, X., Yu, G., Huang, X. et al. (Super)alkali atoms interacting with the σ electron cloud: a novel interaction mode triggers large nonlinear optical response of M@P4 and M@C3H6 (M=Li, Na, K and Li3O). J Mol Model 19, 5601–5610 (2013). https://doi.org/10.1007/s00894-013-2041-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-2041-3