Abstract
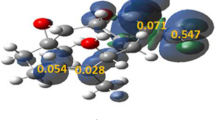
The selectivity of the intramolecular cyclizations of a series of 2’–aminochalcones was investigated with an approach that combines spin–polarized conceptual density functional theory and energy calculations. To that aim, condensed–to–atoms electrophilic Fukui functions, f + NN (r), were utilized as descriptors of the proclivity for nucleophilic attack of the NH2 group on the unsaturated α and β carbons. The results of our model are in excellent agreement with the experimental available evidence permitting us in all cases to predict when the cyclization processes led to the formation of 5–exo and 6–endo products.

Understanding the unexpected cyclizations of 2'aminochalcones


Similar content being viewed by others
References
Go ML, Wu X, Liu XL (2005) Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 12(4):481–499
Dimmock JR, Elias DW, Beazely MA, Kandepu NM (1999) Bioactivities of chalcones. Curr Med Chem 6(12):1125–1149
Katsori AM, Hadjipavlou-Litina D (2009) Chalcones in cancer: understanding their role in terms of qsar. Curr Med Chem 16(9):1062–1081
Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL (1998) Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron 54(16):4085–4096
Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yanez M, Orallo F, Ortuso F, Alcaro S (2009) Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem 52(9):2818–2824
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42(2):125–137
Ahmed N, van Lier JE (2007) Alumina supported-cecl3 center dot 7h(2)onai: an efficient catalyst for the cyclization f 2’–aminochalcones to the corresponding 2–aryl–2,3–dihydroquinolin–4(1h)–ones under solvent free conditions. Tetrahedron Lett 48(1):13–15
Barros A, Silva AMS (2003) One-pot synthesis of 2-(2-hydroxyaryl)quinolines: reductive coupling reactions of 2’–hydroxy–2–nitrochalcones. Tetrahedron Lett 44(31):5893–5896
Bhattacharya RN, Kundu P, Maiti G (2010) Antimony trichloride: an efficient and mild catalyst for cyclization of 2–aminochalcones to the corresponding 2–aryl-2,3-dihydroquinolin-4(1h)-ones. Synth Commun 40(4):476–481
Ahmed N, Kumar H, Babu BV (2013) Intramolecular aminolysis of 2’–aminochalcone epoxides using inbr3 or bicl3 as efficient catalysts. Synth Commun 43(4):567–581
Chelghoum M, Bahnous M, Bouraiou A, Bouacida S, Belfaitah A (2012) An efficient and rapid intramolecular aza-michael addition of 2’–aminochalcones using ionic liquids as recyclable reaction media. Tetrahedron Lett 53(42):4059–4061
Kahriman N, Iskender NY, Yucel M, Yayli N, Demir E, Demirbag Z (2012) Microwave–assisted synthesis of 1,3’–diaza–flavanone/flavone and their alkyl derivatives with antimicrobial activity. J Heterocycl Chem 49(1):71–79
Kumar D, Patel G, Mishra BG, Varma RS (2008) Eco–friendly polyethylene glycol promoted michael addition reactions of alpha, beta–unsaturated carbonyl compounds. Tetrahedron Lett 49(49):6974–6976
Kumar P, Dhar DN (1995) Cyclization of 2’–aminochalcones with chlorosulfonyl isocyanate. Synth Commun 25(13):1933–1938
Li J, Jin L, Yu C, Su W (2009) The cyclisation of 2’–aminochalcones using silica-supported yb(otf)(3) under solvent-free conditions. J Chem Res 3:170–173
Rao VK, Rao MS, Kumar A (2011) Ytterbium(iii) triflate: an efficient and simple catalyst for isomerization of 2’–hydroxychalcone and 2’– aminochalcones in ionic liquid. J Heterocycl Chem 48(6):1356–1360
Yang C, Fang L, Wu L, Yan F (2010) Synthesis of 2–aryl–2,3–dihydroquinolin–4(1h)–ones using wet cyanuric chloride under solvent-free conditions. Asian J Chem 22(8):6031–6034
Abonia R, Cuervo P, Insuasty B, Quiroga J, Nogueras M, Cobo J, Meier H, Lotero E (2008) An amberlyst–(r)–15 mediated synthesis of new functionalized dioxoloquinolinone derivatives. Open J Org Chem 2:26–34
Baldwin JE (1976) Rules for ring-closure. J Chem Soc Chem Comm (18):734–736
Carey FA, Sundberg RJ (1990) Advanced organic chemistry/Francis A. Carey and Richard J. Sundberg, 3rd edn. Plenum, New York
Abonia R, Cuervo P, Castillo J, Insuasty B, Quiroga J, Nogueras M, Cobo J (2008) Unexpected intramolecular cyclization of some 2’–aminochalcones to indolin–3–ones mediated by amberlyst (r)–15. Tetrahedron Lett 49(34):5028–5031
Parr RG, Weitao Y (1994) Density–functional theory of atoms and molecules. Oxford University Press, Oxford
Perez P, Chamorro E, Ayers PW (2008) Universal mathematical identities in density functional theory: results from three different spin–resolved representations. J Chem Phys 128(20):204108–204108
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793–1873
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20(1):129–154
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106(6):2065–2091
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107(9):PR46–PR74
Anderson JSM, Melin J, Ayers PW (2007) Conceptual density-functional theory for general chemical reactions, including those that are neither charge- nor frontier-orbital-controlled. 2. Application to molecules where frontier molecular orbital theory fails. J Chem Theory Comput 3(2):375–389
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101(5):520–534
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122(9):2010–2018
Berkowitz M (1987) Density functional–approach to frontier controlled reactions. J Am Chem Soc 109(16):4823–4825
Yang WT, Parr RG (1985) Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci U S A 82(20):6723–6726
Yang W, Parr RG, Pucci R (1984) Electron–density, kohn-sham frontier orbitals, and Fukui functions. J Chem Phys 81(6):2862–2863
Chattaraj PK, Cedillo A, Parr RG (1995) Variational method for determining the Fukui function and chemical hardness of an electronic system. J Chem Phys 103(17):7645–7646
Fukui K (1982) Role of frontier orbitals in chemical–reactions. Science 218(4574):747–754
Inagaki S, Fujimoto H, Fukui K (1976) Orbital mixing rule. J Am Chem Soc 98(14):4054–4061
Fukui K (1982) The role of frontier orbitals in chemical-reactions (nobel lecture). Angew Chem Int Ed Engl 21(11):801–809
Fukui K, Koga N, Fujimoto H (1981) Interaction frontier orbitals. J Am Chem Soc 103(1):196–197
Galvan M, Vela A, Gazquez JL (1988) Chemical-reactivity in spin-polarized density functional theory. J Phys Chem 92(22):6470–6474
Ghanty TK, Ghosh SK (1994) Spin–polarized generalization of the concepts of electronegativity and hardness and the description of chemical–binding. J Am Chem Soc 116(9):3943–3948
Galvan M, Vargas R (1992) Spin potential in kohn sham theory. J Phys Chem 96(4):1625–1630
Chamorro E, De Proft F, Geerlings P (2005) Hardness and softness reactivity kernels within the spin-polarized density–functional theory. J Chem Phys 123(15):154104–154104
Chamorro E, De Proft F, Geerlings P (2005) Generalized nuclear fukui functions in the framework of spin–polarized density–functional theory. J Chem Phys 123(8):084104–084104
Chamorro E, Perez P (2005) Condensed–to–atoms electronic fukui functions within the framework of spin-polarized density–functional theory. J Chem Phys 123(11):114107–114107
Chamorro E, Perez P, De Proft F, Geerlings P (2006) Philicity indices within the spin-polarized density-functional theory framework. J Chem Phys 124(4):044105–044105
Chamorro E, Perez P, Duque M, De Proft F, Geerlings P (2008) Dual descriptors within the framework of spin-polarized density functional theory. J Chem Phys 129(6):064117–064117
Pinter B, De Proft F, Van Speybroeck V, Hemelsoet K, Waroquier M, Chamorro E, Veszpremi T, Geerlings P (2007) Spin–polarized conceptual density functional theory study of the regioselectivity in ring closures of radicals. J Org Chem 72(2):348–356
Dangate PS, Salunke CL, Akamanchi KG (2011) Regioselective oxidation of cholic acid and its 7 beta epimer by using o–iodoxybenzoic acid. Steroids 76(12):1397–1399
Klaic L, Trippier PC, Mishra RK, Morimoto RI, Silverman RB (2011) Remarkable stereospecific conjugate additions to the hsp90 inhibitor celastrol. J Am Chem Soc 133(49):19634–19637
Peng Z, Pal A, Han D, Wang S, Maxwell D, Levitzki A, Talpaz M, Donato NJ, Bornmann W (2011) Tyrphostin-like compounds with ubiquitin modulatory activity as possible therapeutic agents for multiple myeloma. Bioorg Med Chem 19(23):7194–7204
Parr RG, Bartolotti L (1983) Some remarks on the density functional theory of few-electron systems. J Phys Chem 87(15):2810–2815
Cedillo A (1994) A new representation for ground-states and its legendre transforms. Int J Quantum Chem Suppl 28:231–240
Baekelandt BG, Cedillo A, Parr RG (1995) Reactivity indices and fluctuation formulas in density functional theory: isomorphic ensembles and a new measure of local hardness. J Chem Phys 103(19):8548–8556
De Proft F, Ayers PW, Sen KD, Geerlings P (2004) On the importance pf the “density per particle” (shape function)” in the density functional theory. J Chem Phys 120(21):9969–9973
Contreras RR, Fuentealba P, Galvan M, Perez P (1999) A direct evaluation of regional fukui functions in molecules. Chem Phys Lett 304(5–6):405–413
Fuentealba P, Perez P, Contreras R (2000) On the condensed Fukui function. J Chem Phys 113(7):2544–2551
Bulat FA, Chamorro E, Fuentealba P, Toro-Labbe A (2004) Condensation of frontier molecular orbital fukui functions. J Phys Chem A 108(2):342–349
Tiznado W, Chamorro E, Contreras R, Fuentealba P (2005) Comparison among four different ways to condense the fukui function. J Chem Phys A 109(14):3220–3224
Ayers PW, Morrison RC, Roy RK (2002) Variational principles for describing chemical reactions: condensed reactivity indices. J Chem Phys 116(20):8731–8744
Bultinck P, Fias S, Van Alsenoy C, Ayers PW, Carbo-Dorca R (2007) Critical thoughts on computing atom condensed Fukui functions. J Chem Phys 127(3):034102–034102
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 revision B.01. Gaussian Inc, Wallingford
Acknowledgments
AR thanks Universidad Nacional for their financial support (grant 201010018545). EC and PP acknowledges the continuous support from Fondecyt (Chile), Grant Nos. 1100277 (EC) and 1100278 (PP), and from the Universidad Andres Bello (UNAB) through Grant No. DI-219-12/N (Núcleo CIMFQ).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fondecyt 1100277, Fondecyt 1100278, DI-UNAB 219-12/N (Nucleo CIMFQ), DIB-UNAL 201010016739
Rights and permissions
About this article
Cite this article
Reyes, A., Cuervo, P.A., Orozco, F. et al. Theoretical investigation of the selectivity in intramolecular cyclizations of some 2’–aminochalcones to dihydroquinolin–8–ones and indolin–3–ones. J Mol Model 19, 3611–3618 (2013). https://doi.org/10.1007/s00894-013-1893-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1893-x