Abstract
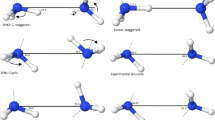
We describe an improved force field parameter set for the generalized AMBER force field (GAFF) for urea. Quantum chemical computations were used to obtain geometrical and energetic parameters of urea dimers and larger oligomers using AM1 semiempirical MO theory, density functional theory at the B3LYP/6-31G(d,p) level, MP2 and CCSD ab initio calculations with the 6-311++G(d,p), aug-cc-pVDZ, aug-cc-pVTZ, and aug-cc-pVQZ basis sets, and with the CBS-QB3 and CBS-APNO complete basis set methods. Seven different urea dimer structures were optimized at the MP2/aug-cc-pVDZ level to obtain accurate interaction energies. Atomic partial charges were calculated at the MP2/aug-cc-pVDZ level with the restrained electrostatic potential (RESP) fitting approach. The interaction energies computed with these new RESP charges in the force field are consistent with those obtained from CCSD and MP2 calculations. The linear dimer structure calculated using the force field with modified geometrical parameters and the new RESP charge set agrees well with available experimental data.








Similar content being viewed by others
References
Bonin M, Marshall WG, Weber HP, Tolendo P (1999) Polymorphism in urea. IOP Publishing, Philadelphia. http://www.isis.rl.ac.uk/archive/isis99/highlights/urea4.htm. Accessed August 1999
Mathews CK, van Holde KE (1996) Biochemistry, 2nd edn. Benjamin Cummings, Menlo Park, p 4
Bhatnagar VM (1968) Clathrates of urea nad thiourea. J Struct Chem 8:513–529. doi:10.1007/BF00751656
Theophanides T, Harvey PD (1987) Structural and spectroscopic properties of metal–urea complexes. Coord Chem Rev 76:237–264. doi:10.1016/0010-8545(87)85005-1
Zavodnik V, Stash A, Tsirelson V, De Vires R, Feil D (1999) Electron density study of urea using TDS-corrected X-ray diffraction data: quantitative comparison of experimental and theoretical results. Acta Crystallogr Sect B 55:45–54. doi:10.1107/S0108768198005746
Vaughan P, Donohue J (1952) The structure of urea. Interatomic distances and resonance in urea and related compounds. Acta Crystallogr 5:530–535. doi:10.1107/S0365110X52001477
Worsham JE, Levy HA, Peterson SE (1957) The positions of hydrogen atoms in urea by neutron diffraction. Acta Crystallogr 10:319–323. doi:10.1107/S0365110X57000924
Andrew ER, Hyndman D (1953) Proton magnetic resonance evidence for the planar structure of the urea molecule. Proc Phys Soc A66:1187–1188. doi:10.1088/0370-1298/66/12/119
Wyckoff RWG (1930) Z Kristallogr 75:529–537
Hendriks SB (1928) The crystal structure of urea and the molecular symmetry of thiourea. J Am Chem Soc 50:2455–2464. doi:10.1021/ja01396a019
Mark H, Weissenberg K (1923) Röntgenographische Bestimmung der Struktur des Harnstoffs und des Zinntetrajodids. Z Phys 16:1–22. doi:10.1007/BF01327372
Sklar N, Senko ME, Post B (1961) Thermal effects in urea: the crystal structure at −140 °C and at room temperature. Acta Crystallogr 14:716–722. doi:10.1107/S0365110X61002187
Swaminathan S, Craven BM (1984) The crystal structure and molecular thermal motion of urea at 12, 60 and 123 K from neutron diffraction. Acta Crystallogr Sect B 40:300–306. doi:10.1107/S0108768184002135
Pluta T, Sadlej AJ (2001) Electric properties of urea and thiourea. J Chem Phys 114:136–146. doi:10.1063/1.1328398
Alparone A, Millefiori S (2005) Gas and solution phase electronic and vibrational (hyper)polarizabilities in the series formaldehyde, formamide and urea: CCSD(T) and DFT theoretical study. Chem Phys Lett 416:282–288
Olah GA, Surya Prakash GK, Rasul G (2008) 13C and 15N NMR and ab initio/GIAO-CCSD(T) study of the structure of mono-, di-, and triprotonated guanidine, urea, and thiourea. J Phys Chem C 112:7895–7899. doi:10.1021/jp711727c
Benková Z, Černušák I, Zahradník P (2007) Electric properties of formaldehyde, thioformaldehyde, urea, formamide, and thioformamide: post-HF and DFT study. Int J Quantum Chem 107:2133–2152. doi:10.1002/qua.21399
Masunov A, Dannenberg JJ (1999) Theoretical study of urea. I. Monomers and dimers. J Phys Chem A 103:178–184. doi:10.1021/jp9835871
Spoliti M, Perrone G, Bencivenni L, Pieretti A, Grandi A, Ramondo F (2005) Computational and vibrational spectroscopy study of the microclusters of C2 symmetry urea molecule in the 1A electronic ground state. J Mol Struct 756:113–126. doi:10.1016/j.theochem.2005.07.021
Singh A, Chakraborty S, Ganguly B (2007) Computational study of urea and its homologue glycinamide: conformations, rotational barriers, and relative interactions with sodium chloride. Langmuir 23:5406–5411. doi:10.1021/la062405o
Civalleri B, Doll K, Zicovich-Wilson CM (2007) Ab initio investigation of structure and cohesive energy of crystalline urea. J Phys Chem B 111:26–33. doi:10.1021/jp065757c
Boek ES, Briels WJ (1993) Molecular dynamics simulations of aqueous urea solutions: study of dimer stability and solution structure, and calculation of the total nitrogen radial distribution function GN(r). J Chem Phys 98:1422–1427. doi:10.1063/1.464306
Astrand PO, Wallqvist A, Karlström G (1994) Molecular dynamics simulations of 2 m aqueous urea solutions. J Phys Chem 98:8224–8233. doi:10.1021/j100084a046
Boek ES, Briels WJ, van Eerden J, Feil D (1992) Molecular-dynamics simulations of interfaces between water and crystalline urea. J Chem Phys 96:7010–7018. doi:10.1063/1.462560
Liu XY, Boek ES, Briels WJ, Bennema P (1995) Analysis of morphology of crystals based on identification of interfacial structure. J Chem Phys 103:3747–3754. doi:10.1063/1.470053
Kallies B (2002) Coupling of solvent and solute dynamics—molecular dynamics simulations of aqueous urea solutions with different intramolecular potentials. Phys Chem Chem Phys 4:86–95. doi:10.1039/b105836n
Caballo-Herrera A, Nilsson L (2006) Urea parametrization for molecular dynamics simulations. J Mol Struct Theochem 758:139–148. doi:10.1016/j.theochem.2005.10.018
Etter M (1990) Encoding and decoding hydrogen-bond patterns of organic compounds. Acc Chem Res 23:120–126. doi:10.1021/ar00172a005
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174. doi:10.1002/jcc.20035
Clark T, Alex A, Beck B, Burkhardt F, Chandrasekhar J, Gedeck P, Horn A, Hutter M, Martin B, Rauhut G, Sauer W, Schindler T, Steinke T (2003) VAMP v.10.0. Accelrys Inc., San Diego
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision D.02. Gaussian, Inc., Wallingford
Čízek J (1969) In: Lefebre R, Moser C (eds) Correlation effects in atoms & molecules. Advances in chemical physics 14. Interscience, New York, pp 35
Purvis GD, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J Chem Phys 76:1910–1918. doi:10.1063/1.443164
Scuseria GE, Janssen CL, Schaefer HF III (1988) An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J Chem Phys 89:7382–7387. doi:10.1063/1.455269
Moller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622. doi:10.1103/PhysRev.46.618
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Dewar M, Thiel W (1977) Ground states of molecules. 38. The MNDO method. Approximations and parameters. J Am Chem Soc 99:4899–4907. doi:10.1021/ja00457a004
Nyden MR, Petersson GA (1981) Complete basis set correlation energies. I. The asymptotic convergence of pair natural orbital expansions. J Chem Phys 75:1843–1862. doi:10.1063/1.442208
Petersson GA, Al-Laham MA (1991) A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J Chem Phys 94:6081–6090. doi:10.1063/1.460447
Petersson GA, Tensfeldt TG, Montgomery JA Jr (1991) A complete basis set model chemistry. III. The complete basis set-quadratic configuration interaction family of methods. J Chem Phys 94:6091–6101. doi:10.1063/1.460448
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. doi:10.1063/1.456153
Kendall RA, Dunning TH Jr, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806. doi:10.1063/1.462569
Woon DE, Dunning TH Jr (1993) Gaussian-basis sets for use in correlated molecular calculations. 3. The atoms aluminum through argon. J Chem Phys 98:1358–1371. doi:10.1063/1.464303
Peterson KA, Woon DE, Dunning TH Jr (1994) Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+H2→H2+H reaction. J Chem Phys 100:7410–7415. doi:10.1063/1.466884
Wilson AK, van Mourik T, Dunning TH Jr (1996) Gaussian basis sets for use in correlated molecular calculations. VI. Sextuple zeta correlation consistent basis sets for boron through neon. J Mol Struct 388:339–349. doi:10.1016/S0166-1280(96)80048-0
McLean AD, Chandler GS (1980) Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z=11–18. J Chem Phys 72:5639–5648. doi:10.1063/1.438980
Raghavachari K, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. 20. Basis set for correlated wave-functions. J Chem Phys 72:650–654. doi:10.1063/1.438955
Clark T, Chandrasekhar J, Spitznagel GW, PvR S (1983) Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21+G basis set for 1st-row elements, Li-F. J Comput Chem 4:294–301. doi:10.1002/jcc.540040303
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular orbital methods. 9. Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728. doi:10.1063/1.1674902
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods. 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269. doi:10.1063/1.447079
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. doi:10.1080/00268977000101561
Case DA, Darden TA, Cheatham TE III, Simmerling CL, Wang J, Duke RE, Luo R, Crowley M, Walker RC, Zhang W, Merz KM, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Mathews DH, Seetin MG, Sagui C, Babin V, Kollman PA (2008) AMBER 10. University of California, San Francisco
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280. doi:10.1021/j100142a004
Brown RD, Godfrey PD, Story J (1975) The microwave spectrum of urea. J Mol Spectrosc 58:445–450. doi:10.1016/0022-2852(75)90224-6
Strassner T (1996) Ab initio and molecular mechanics calculations of various substituted ureas: rotational barriers and a new parametrization for ureas. J Mol Model 2:217–226. doi:10.1007/s0089460020217
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197. doi:10.1021/ja00124a002
Gilkerson W, Srivastava K (1960) The dipole moment of urea. J Phys Chem 64:1485–1487. doi:10.1021/j100839a032
Lefebvre J (1973) Longitudinal phonons of translation along the 4 axis on urea. Solid State Commun 13:1873–1875. doi:10.1016/0038-1098(73)90748-5
Lee JS (1999) On the effectiveness of function counterpoise method in the calculation of He–He interaction energies. Bull Korean Chem Soc 20:241–243
Schwenke DW, Truhlar DG (1985) Systematic study of basis set superposition errors in the calculated interaction energy of two HF molecules. J Chem Phys 85:2418–2426. doi:10.1063/1.448335
Åstrand PO, Wallqvist A, Karlström G (1994) Nonempirical intermolecular potentials for urea–water systems. J Chem Phys 100:1262–1273. doi:10.1063/1.466655
Suzuki K, Onishi S, Koide T, Seki S (1956) Vapor pressures of molecular crystals. XI. Vapor pressures of crystalline urea and diformylhydrazine. Energies of hydrogen bonds in these crystals. Bull Chem Soc Jpn 29:127–131. doi:10.1246/bcsj.29.127
Dewit HGM, Van Miltenburg JC, DeKruif CG (1983) Thermodynamic properties of molecular organic crystals containing nitrogen, oxygen, and sulphur 1. Vapour pressures and enthalpies of sublimation. J Chem Thermodyn 15:651–663. doi:10.1016/002-9614(83)90079-4
Bertran CA, Cirino JJV, Freitas LCG (2002) Theoretical studies of concentrated aqueous urea solutions using computational Monte Carlo simulation. J Braz Chem Soc 13:238–244. doi:10.1590/S0103-50532002000200016
Duffy EM, Severance DL, Jorgensen WL (1993) Urea: potential functions, log P, and free energy of hydration. Isr J Chem 33:323–330
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft as part of the project PE 42710-2 and the Excellence Cluster Engineering of Advanced Materials. We also thank Matthias Hennemann, Christof Jäger and Frank Beierlein for their support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material
(DOC 687 kb)
Rights and permissions
About this article
Cite this article
Özpınar, G.A., Peukert, W. & Clark, T. An improved generalized AMBER force field (GAFF) for urea. J Mol Model 16, 1427–1440 (2010). https://doi.org/10.1007/s00894-010-0650-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0650-7