Abstract
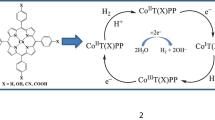
Cobalt porphyrins have been shown to electrocatalyze O2 reduction as well as H2 evolution. A cobalt porphyrin is synthesized when two electron-withdrawing groups are used in an attempt to reduce the overpotential involved in O2 reduction and H2 evolution. The results show that in the case of O2 reduction, instead of reduction of overpotential, the selectivity for O2 reduction changes to 2e−/2H+ to produce H2O2. In the case of H2 evolution, the CoI state is incapable of catalyzing H+ reduction. Rather, a CoIII–H species needs to be reduced to CoII–H before H2 can be produced. There results add to the ongoing investigations into the factors that control the rates, overpotential and selectivity of these important cathodic processes.







Similar content being viewed by others
References
Zhang W, Lai W, Cao R (2017) Chem Rev 117:3717–3797
Dey S, Mondal B, Chatterjee S, Rana A, Amanullah S, Dey A (2017) Nat Rev Chem 1:0098
Kärkäs MD, Verho O, Johnston EV, Åkermark B (2014) Chem Rev 114:11863–12001
McEvoy JP, Brudvig GW (2006) Chem Rev 106:4455–4483
Pegis ML, Wise CF, Martin DJ, Mayer JM (2018) Chem Rev 118:2340–2391
Holm RH, Kennepohl P, Solomon EI (1996) Chem Rev 96:2239–2314
Amanullah S, Singha A, Dey A (2019) Coord Chem Rev 386:183–208
Kim E, Chufán EE, Kamaraj K, Karlin KD (2004) Chem Rev 104:1077–1134
Collman JP, Boulatov R, Sunderland CJ, Fu L (2004) Chem Rev 104:561–588
Adam SM, Wijeratne GB, Rogler PJ, Diaz DE, Quist DA, Liu JJ, Karlin KD (2018) Chem Rev 118:10840–11022
Sengupta K, Chatterjee S, Samanta S, Dey A (2013) Proc Natl Acad Sci 110:8431
Chatterjee S, Sengupta K, Mondal B, Dey S, Dey A (2017) Acc Chem Res 50:1744–1753
Bhunia S, Rana A, Roy P, Martin DJ, Pegis ML, Roy B, Dey A (2018) J Am Chem Soc 140:9444–9457
Bhugun I, Lexa D, Savéant J-M (1996) J Am Chem Soc 118:1769–1776
Schöfberger W, Faschinger F, Chattopadhyay S, Bhakta S, Mondal B, Elemans JAAW, Müllegger S, Tebi S, Koch R, Klappenberger F, Paszkiewicz M, Barth JV, Rauls E, Aldahhak H, Schmidt WG, Dey A (2016) Angew Chem Int Ed 55:2350–2355
Rana A, Mondal B, Sen P, Dey S, Dey A (2017) Inorg Chem 56:1783–1793
Mondal B, Sen P, Rana A, Saha D, Das P, Dey A (2019) ACS Catal 9:3895–3899
Trasatti S (1986) Pure Appl Chem 58:955
Armstrong DA, Huie RE, Koppenol WH, Lymar SV, Merényi G, Neta P, Ruscic B, Stanbury DM, Steenken S, Wardman P (2015) Pure Appl Chem 87(11–12):1139–1150
Bratsch SG (1989) J Phys Chem Ref Data 18:1–21
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, Valentine JS (2014) Chem Rev 114:3854–3918
D’Souza F, Hsieh Y-Y, Deviprasad GR (1997) J Electroanal Chem 426:17–21
Amanullah S, Das PK, Samanta S, Dey A (2015) Chem Commun 51:10010–10013
Grinstaff MW, Hill MG, Birnbaum ER, Schaefer WP, Labinger JA, Gray HB (1995) Inorg Chem 34:4896–4902
Mahammed A, Mondal B, Rana A, Dey A, Gross Z (2014) Chem Commun 50:2725–2727
Mondal B, Sengupta K, Rana A, Mahammed A, Botoshansky M, Dey SG, Gross Z, Dey A (2013) Inorg Chem 52:3381–3387
Passard G, Ullman AM, Brodsky CN, Nocera DG (2016) J Am Chem Soc 138:2925–2928
Pegis ML, McKeown BA, Kumar N, Lang K, Wasylenko DJ, Zhang XP, Raugei S, Mayer JM (2016) ACS Central Sci 2:850–856
Pegis ML, Wise CF, Koronkiewicz B, Mayer JM (2017) J Am Chem Soc 139:11000–11003
Amanullah S, Saha P, Saha R, Dey A (2019) Inorg Chem 58:152–164
Savéant J-M (2006) Elements of molecular and biomolecular electrochemistry: an electrochemical approach to electron transfer chemistry, Wiley, New York
Chatterjee S, Sengupta K, Samanta S, Das PK, Dey A (2015) Inorg Chem 54:2383–2392
McCrory CCL, Ottenwaelder X, Stack TDP, Chidsey CED (2007) J Phys Chem A 111:12641–12650
McCrory CCL, Devadoss A, Ottenwaelder X, Lowe RD, Stack TDP, Chidsey CED (2011) J Am Chem Soc 133:3696–3699
Thorseth MA, Letko CS, Rauchfuss TB, Gewirth AA (2011) Inorg Chem 50:6158–6162
Lee CH, Dogutan DK, Nocera DG (2011) J Am Chem Soc 133:8775–8777
Acknowledgements
This research was funded by the Council of Scientific and Industrial Research (CSIR) Grant no. 01(2874)/17/EMR-II. S.A. acknowledges CSIR-SRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amanullah, S., Dey, A. A bi-functional cobalt-porphyrinoid electrocatalyst: balance between overpotential and selectivity. J Biol Inorg Chem 24, 437–442 (2019). https://doi.org/10.1007/s00775-019-01670-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01670-5