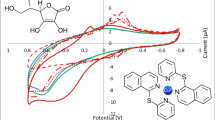
Abstract
Amyloid-β peptides and their metal-associated aggregated states have been implicated in the pathogenesis of Alzheimer’s disease. The present paper epitomises the design and synthesis of a small, neutral, lipophilic benzothiazole Schiff base (E)-2-((6-chlorobenzo[d]thiazol-2-ylimino)methyl)-5-diethylamino)phenol (CBMDP), and explores its multifunctionalty as a potential metal chelator/fluorophore using UV–visible absorption, steady-state fluorescence, single molecule fluorescence correlation spectroscopic (FCS) techniques which is further corroborated by in silico studies. Some pharmaceutically relevant properties of the synthesized compound have also been calculated theoretically. Steady-state fluorescence and single molecule FCS reveal that the synthesized CBMDP not only recognizes oligomeric Aβ40, but could also be used as an amyloid-specific extrinsic fluorophore as it shows tremendous increase in its emission intensity in the presence of Aβ40. Molecular docking exercise and MD simulation reveal that CBMDP localizes itself in the crucial amyloidogenic and copper-binding region of Aβ40 and undergoes a strong binding interaction via H-bonding and π–π stacking. It stabilizes the solitary α-helical Aβ40 monomer by retaining the initial conformation of the Aβ central helix and mostly interacts with the hydrophilic N-terminus and the α-helical region spanning from Ala-2 to Val-24. CBMDP exhibits strong copper as well as zinc chelation ability and retards the rapid copper-induced aggregation of amyloid peptide. In addition, CBMDP shows radical scavenging activity which enriches its functionality. Overall, the consolidated in vitro and in silico results obtained for the synthesized molecule could provide a rational template for developing new multifunctional agents.









Similar content being viewed by others
References
Wang Z-M, Xie S-S, Li X-M et al (2015) Multifunctional 3-Schiff base-4-hydroxycoumarin derivatives with monoamine oxidase inhibition, anti-β-amyloid aggregation, metal chelation, antioxidant and neuroprotection properties against Alzheimer’s disease. RSC Adv 5:70395–70409. doi:10.1039/C5RA13594J
Yanagisawa S (2012) Metal complexes for optical recording material, JP 5036190
Choudhury S, Kakoti M, Deb AK, Goswami S (1992) Isomeric complexes of ruthenium(II) with neutral heterocyclic Schiff base ligands. High resolution proton resonance spectra of trans-cis isomeric pairs of RuX2L2 (L = 2-arylpyridinecarboxaldimine, X = Cl, Br) and comparison of their physical properties. Polyhedron 11:3183–3190. doi:10.1016/S0277-5387(00)83661-X
Sarkar B, Konar S, Gómez-García CJ, Ghosh A (2008) Rare example of mu-nitrito-1kappa2O, O’:2kappaO coordinating mode in copper(II) nitrite complexes with monoanionic tridentate Schiff base ligands: structure, magnetic, and electrochemical properties. Inorg Chem 47:11611–11619. doi:10.1021/ic8011519
Heffern MC, Velasco PT, Matosziuk LM et al (2014) Modulation of amyloid-β aggregation by histidine-coordinating Cobalt(III) Schiff base complexes. ChemBioChem 15:1584–1589. doi:10.1002/cbic.201402201
Ghosh C, Seal M, Mukherjee S, Ghosh Dey S (2015) Alzheimer’s disease: a heme-Aβ perspective. Acc Chem Res 48:2556–2564. doi:10.1021/acs.accounts.5b00102
Association Alzheimer's (2016) 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 12(4):459–509. doi:10.1016/j.jalz.2016.03.001
Sengupta P, Garai K, Sahoo B et al (2003) The amyloid beta peptide (Abeta(1-40)) is thermodynamically soluble at physiological concentrations. Biochemistry 42:10506–10513. doi:10.1021/bi0341410
Rauk A (2009) The chemistry of Alzheimer’s disease. Chem Soc Rev 38:2698–2715. doi:10.1039/b807980n
Davies P, Maloney AJF (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 308:1403. doi:10.1016/S0140-6736(76)91936-X
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Butterfield DA (1997) beta-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem Res Toxicol 10:495–506. doi:10.1021/tx960130e
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71:621s–629s
Sengupta K, Chatterjee S, Pramanik D et al (2014) Self-assembly of stable oligomeric and fibrillar aggregates of Aβ peptides relevant to Alzheimer’s disease: morphology dependent Cu/heme toxicity and inhibition of PROS generation. Dalton Trans 43:13377–13383. doi:10.1039/c4dt01991a
Bonda DJ, Lee H, Blair JA et al (2011) Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics 3:267–270. doi:10.1039/c0mt00074d
Dubois B, Feldman HH, Jacova C et al (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–1127. doi:10.1016/S1474-4422(10)70223-4
Kayed R, Lasagna-Reeves C (2013) Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis 33:S67–S78. doi:10.3233/JAD-2012-129001
Jarosz-Griffiths HH, Noble E, Rushworth JV, Hooper NM (2016) Amyloid-β receptors: the good, the bad, and the prion protein. J Biol Chem 291:3174–3183. doi:10.1074/jbc.R115.702704
Masters CL, Simms G, Weinman NA et al (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome 82:4245–4249
Hane FT, Lee BY, Petoyan A et al (2014) Testing synthetic amyloid-β aggregation inhibitor using single molecule atomic force spectroscopy. Biosens Bioelectron 54:492–498. doi:10.1016/j.bios.2013.10.060
Hane FT, Leonenko Z (2014) Effect of Metals on Kinetic Pathways of Amyloid-β Aggregation. Biomolecules 4:101–116. doi:10.3390/biom4010101
Balland V, Hureau C, Saveant J-M (2010) Electrochemical and homogeneous electron transfers to the Alzheimer amyloid–copper complex follow a preorganization mechanism. Proc Natl Acad Sci 107:17113–17118. doi:10.1073/pnas.1011315107
Sharma AK, Pavlova ST, Kim J et al (2012) Bifunctional compounds for controlling metal-mediated aggregation of the aβ42 peptide. J Am Chem Soc 134:6625–6636. doi:10.1021/ja210588m
Hindo SS, Mancino AM, Braymer JJ et al (2009) Small molecule modulators of copper-induced Aβ aggregation. J Am Chem Soc 131:16663–16665. doi:10.1021/ja907045h
Rodríguez-Rodríguez C, Sánchez de Groot N, Rimola A et al (2009) Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J Am Chem Soc 131:1436–1451
León R, Garcia AG, Marco-Contelles J (2013) Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med Res Rev 33:139–189. doi:10.1002/med.20248
Cavalli A, Bolognesi ML, Minarini A et al (2008) Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem 51:347–372. doi:10.1021/jm7009364
Braymer JJ, Choi JS, Detoma AS et al (2011) Development of bifunctional stilbene derivatives for targeting and modulating metal-amyloid-β species. Inorg Chem 50:10724–10734. doi:10.1021/ic2012205
Choi J-S, Braymer JJ, Nanga RPR et al (2010) Design of small molecules that target metal-A{beta} species and regulate metal-induced A{beta} aggregation and neurotoxicity. Proc Natl Acad Sci USA 107:21990–21995. doi:10.1073/pnas.1006091107
Lee S, Zheng X, Krishnamoorthy J et al (2014) Rational design of a structural framework with potential use to develop chemical reagents that target and modulate multiple facets of Alzheimer’s disease. J Am Chem Soc 136:299–310. doi:10.1021/ja409801p
Sengupta P, Balaji J, Maiti S (2002) Measuring diffusion in cell membranes by fluorescence correlation spectroscopy. Methods 27:374–387. doi:10.1016/S1046-2023(02)00096-8
Halgren TA, Murphy RB, Friesner RA et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759. doi:10.1021/jm030644s
Sahoo B, Balaji J, Nag S et al (2008) Protein aggregation probed by two-photon fluorescence correlation spectroscopy of native tryptophan. J Chem Phys 129:075103. doi:10.1063/1.2969110
Garai K, Sureka R, Maiti S (2007) Detecting amyloid-beta aggregation with fiber-based fluorescence correlation spectroscopy. Biophys J 92:L55–L57. doi:10.1529/biophysj.106.101485
Klein SM, Cohen G, Cederbaum AI (1981) Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 20:6006–6012. doi:10.1021/bi00524a013
Darghal N, Garnier-Suillerot A, Salerno M (2006) Mechanism of thioflavin T accumulation inside cells overexpressing P-glycoprotein or multidrug resistance-associated protein: role of lipophilicity and positive charge. Biochem Biophys Res Commun 343:623–629. doi:10.1016/j.bbrc.2006.03.024
Begley D (2004) ABC transporters and the blood–brain barrier. Curr Pharm Des 10:1295–1312. doi:10.2174/1381612043384844
Mathis CA, Bacskai BJ, Kajdasz ST et al (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. doi:10.1016/S0960-894X(01)00734-X
Nesterov EE, Skoch J, Hyman BT et al (2005) In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chemie Int Ed 44:5452–5456. doi:10.1002/anie.200500845
Ioakimidis L, Thoukydidis L, Mirza A et al (2008) Benchmarking the reliability of QikProp. Correlation between experimental and predicted values. QSAR Comb Sci 27:445–456. doi:10.1002/qsar.200730051
Carrico D, Ohkanda J, Kendrick H et al (2004) In vitro and in vivo antimalarial activity of peptidomimetic protein farnesyltransferase inhibitors with improved membrane permeability. Bioorg Med Chem 12:6517–6526. doi:10.1016/j.bmc.2004.09.020
Löscher W, Potschka H (2005) Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6:591–602. doi:10.1038/nrn1728
Löscher W, Potschka H (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86–98. doi:10.1602/neurorx.2.1.86
Telpoukhovskaia MA, Rodríguez-Rodríguez C, Cawthray JF et al (2014) 3-hydroxy-4-pyridinone derivatives as metal ion and amyloid binding agents. Metallomics 6:249–262. doi:10.1039/c3mt00135k
LeVine H (1999) Amyloid, prions, and other protein aggregates. Methods Enzymol 309:274–284. doi:10.1016/S0076-6879(99)09020-5
Kirschner DA, Inouye H, Duffy LK et al (1987) Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc Natl Acad Sci USA 84:6953–6957. doi:10.1073/pnas.84.19.6953
Wood SJ, Wetzel R, Martin JD, Hurle MR (1995) Prolines and amyloidogenicity in fragments of the Alzheimer’s peptide beta/A4. Biochemistry 34:724–730
Garbuzynskiy SO, Lobanov MY, Galzitskaya OV (2010) FoldAmyloid: a method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 26:326–332. doi:10.1093/bioinformatics/btp691
Sticht H, Bayer P, Willbold D et al (1995) Structure of amyloid A4-(1-40)-peptide of Alzheimer’s disease. Eur J Biochem 233:293–298. doi:10.1111/j.1432-1033.1995.293_1.x
Kepp KP (2012) Bioinorganic chemistry of Alzheimer’s disease. Chem Rev 112:5193–5239. doi:10.1021/cr300009x
Miller Y, Ma B, Nussinov R (2012) Metal binding sites in amyloid oligomers: complexes and mechanisms. Coord Chem Rev 256:2245–2252. doi:10.1016/j.ccr.2011.12.022
Xu L, Wang X, Wang X (2013) Effects of Zn 2+ binding on the structural and dynamic properties of amyloid Β peptide associated with Alzheimer’s disease: Asp 1 or Glu 11 ? ACS Chem Neurosci 4:1458–1468. doi:10.1021/cn4001445
Ito M, Johansson J, Strömberg R, Nilsson L (2012) Effects of ligands on unfolding of the amyloid β-peptide central helix: mechanistic insights from molecular dynamics simulations. PLoS ONE 7:e30510. doi:10.1371/journal.pone.0030510
Xu Y, Shen J, Luo X et al (2005) Conformational transition of amyloid beta-peptide. Proc Natl Acad Sci USA 102:5403–5407. doi:10.1073/pnas.0501218102
Raffa DF, Rauk A (2007) Molecular dynamics study of the beta amyloid peptide of Alzheimer’s disease and its divalent copper complexes. J Phys Chem B 111:3789–3799. doi:10.1021/jp0689621
Geng J, Li M, Wu L et al (2012) Liberation of copper from amyloid plaques: making a risk factor useful for Alzheimer’s disease treatment 55:9146–9155
Hane FT, Hayes R, Lee BY, Leonenko Z (2016) Effect of copper and zinc on the single molecule self-affinity of Alzheimer’s amyloid-β peptides. PLoS ONE 11:e0147488. doi:10.1371/journal.pone.0147488
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Jadhao M, Ahirkar P, Kumar H et al (2015) Surfactant induced aggregation–disaggregation of photodynamic active chlorin e6 and its relevant interaction with DNA alkylating quinone in a biomimic micellar microenvironment. RSC Adv 5:81449–81460. doi:10.1039/C5RA16181A
Sharma AK, Kim J, Prior JT et al (2014) Small bifunctional chelators that do not disaggregate amyloid β fibrils exhibit reduced cellular toxicity. Inorg Chem 53:11367–11376
Lovell M, Robertson J, Teesdale W et al (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52. doi:10.1016/S0022-510X(98)00092-6
Huang X, Atwood CS, Hartshorn MA et al (1999) The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38:7609–7616. doi:10.1021/bi990438f
Huang X, Cuajungco MP, Atwood CS et al (1999) Cu(II) potentiation of Alzheimer a neurotoxicity: correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem 274:37111–37116. doi:10.1074/jbc.274.52.37111
Mayes J, Tinker-Mill C, Kolosov O et al (2014) Amyloid fibrils in alzheimer disease are not inert when bound to copper ions but can degrade hydrogen peroxide and generate reactive oxygen species. J Biol Chem 289:12052–12062. doi:10.1074/jbc.M113.525212
Gutteridge JMC (1984) Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett 172:245–249. doi:10.1016/0014-5793(84)81134-5
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214. doi:10.1096/fj.02-0752rev
Xu G, Chance MR (2007) Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem Rev 107:3514–3543. doi:10.1021/cr0682047
Acknowledgements
SKG gratefully acknowledges the financial support from the Department of Science and Technology, India (Project no. SR/FT/LS/-172/2009) MJ thanks VNIT for his fellowship. The authors thank G. Krishnamoorthy, TIFR Mumbai, for providing steady-state fluorescence facility. The authors appreciate and thank the anonymous reviewers and the respected editor for their kind suggestions to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jadhao, M., Das, C., Rawat, A. et al. Development of multifunctional heterocyclic Schiff base as a potential metal chelator: a comprehensive spectroscopic approach towards drug discovery. J Biol Inorg Chem 22, 47–59 (2017). https://doi.org/10.1007/s00775-016-1407-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1407-2