Abstract
Introduction
Existing osteoporosis models in sheep exhibit some disadvantages, e.g., challenging surgical procedures, serious ethical concerns, failure of reliable induction of substantial bone loss, or lack of comparability to the human condition. This study aimed to compare bone morphological and mechanical properties of old and young sheep, and to evaluate the suitability of the old sheep as a model for senile osteopenia.
Materials and methods
The lumbar vertebral body L3 of female merino sheep with two age ranges, i.e., old animals (6–10 years; n = 41) and young animals (2–4 years; n = 40), was analyzed concerning its morphological and mechanical properties by bone densitometry, quantitative histomorphometry, and biomechanical testing of the corticalis and/or central spongious region.
Results
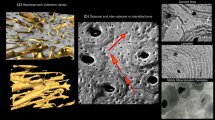
In comparison with young sheep, old animals showed only marginally diminished bone mineral density of the vertebral bodies, but significantly decreased structural (bone volume, − 15.1%; ventral cortical thickness, − 11.8%; lateral cortical thickness, − 12.2%) and bone formation parameters (osteoid volume, osteoid surface, osteoid thickness, osteoblast surface, all − 100.0%), as well as significantly increased bone erosion (eroded surface, osteoclast surface). This resulted in numerically decreased biomechanical properties (compressive strength; − 6.4%).
Conclusion
Old sheep may represent a suitable model of senile osteopenia with markedly diminished bone structure and formation, and substantially augmented bone erosion. The underlying physiological aging concept reduces challenging surgical procedures and ethical concerns and, due to complex alteration of different facets of bone turnover, may be well representative of the human condition.







Similar content being viewed by others
References
Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, Harris ST, Jan de Beur SM, Khosla S, Lane NE, Lindsay R, Nana AD, Orwoll ES, Saag K, Silverman S, Watts NB (2014) The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporosis Int 25:1439–1443
Kanis J (2007) Assessment of osteoporosis at the primary health-care level. Technical report. University of Sheffield, Sheffield
Thompson DD, Simmons HA, Pirie CM, Ke HZ (1995) FDA Guidelines and animal models for osteoporosis. Bone 17:125s–133s
Chappard D, Legrand E, Basle MF, Fromont P, Racineux JL, Rebel A, Audran M (1996) Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Min Res 11:676–685
Rocca M, Fini M, Giavaresi G, Aldini NN, Giardino R (2002) Osteointegration of hydroxyapatite-coated and uncoated titanium screws in long-term ovariectomized sheep. Biomaterials 23:1017–1023
Sachse A, Wagner A, Keller M, Wagner O, Wetzel WD, Layher F, Venbrocks RA, Hortschansky P, Pietraszczyk M, Wiederanders B, Hempel HJ, Bossert J, Horn J, Schmuck K, Mollenhauer J (2005) Osteointegration of hydroxyapatite-titanium implants coated with nonglycosylated recombinant human bone morphogenetic protein-2 (BMP-2) in aged sheep. Bone 37:699–710
Chavassieux P, Vergnaud P, Garnero P, Meunier P (1997) Short-term effects of corticosteroids on trabecular bone remodeling in old ewes. Bone 20:451–455
Turner AS (2002) The sheep as a model for osteoporosis in humans. Vet J (London, England: 1997) 163:232–239
Oheim R, Amling M, Ignatius A, Pogoda P (2012) Large animal model for osteoporosis in humans: the ewe. Eur Cells Mater 24:372–385
Aerssens J, Boonen S, Lowet G, Dequeker J (1998) Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139:663–670
Karasov WH, Douglas AE (2013) Comparative digestive physiology. Compr Physiol 3:741–783
Wilke HJ, Kettler A, Wenger KH, Claes LE (1997) Anatomy of the sheep spine and its comparison to the human spine. Anat Rec 247:542–555
Bungartz M, Maenz S, Kunisch E, Horbert V, Xin L, Gunnella F, Mika J, Borowski J, Bischoff S, Schubert H, Sachse A, Illerhaus B, Gunster J, Bossert J, Jandt KD, Kinne RW, Brinkmann O (2016) First-time systematic postoperative clinical assessment of a minimally invasive approach for lumbar ventrolateral vertebroplasty in the large animal model sheep. Spine J 16:1263–1275
Turner AS, Alvis M, Myers W, Stevens ML, Lundy MW (1995) Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone 17:395s–402s
MacLeay JOJ, Enns R, Les C, Toth C, Wheeler D, Turner A (2004) Dietary-induced metabolic acidosis decreases bone mineral density in mature ovariectomized ewes. Calcif Tissue Int 75:431–437
Macleay JM, Olson JD, Turner AS (2004) Effect of dietary-induced metabolic acidosis and ovariectomy on bone mineral density and markers of bone turnover. J Bone Miner Metab 22:561–568
Kennedy OD, Brennan O, Mahony NJ, Rackard SM, O'Brien FJ, Taylor D, Lee CT (2008) Effects of high bone turnover on the biomechanical properties of the L3 vertebra in an ovine model of early stage osteoporosis. Spine 33:2518–2523
Wu ZX, Lei W, Hu YY, Wang HQ, Wan SY, Ma ZS, Sang HX, Fu SC, Han YS (2008) Effect of ovariectomy on BMD, micro-architecture and biomechanics of cortical and cancellous bones in a sheep model. Med Eng Phys 30:1112–1118
Oheim R, Beil FT, Kohne T, Wehner T, Barvencik F, Ignatius A, Amling M, Clarke IJ, Pogoda P (2013) Sheep model for osteoporosis: sustainability and biomechanical relevance of low turnover osteoporosis induced by hypothalamic-pituitary disconnection. J Orthop Res 31:1067–1074
Kreipke NCRTC, Garrison JG, Easley JT, Turner AS, Niebur GL (2014) Alterations in trabecular bone microarchitecture in the ovine spine and distal femur following ovariectomy. J Biomech 47:1918–1921
Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, Amling M (2006) Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res 21:1591–1599
Lill AKFCA, Schneider E (2002) Effect of ovariectomy malnutrition and glucocorticoid application on bone properties in sheep: a pilot study. Osteoporos Int 13:480–486
Ding M, Bollen P, Schwarz P, Overgaard S (2010) Glucocorticoid induced osteopenia in cancellous bone of sheep. Spine 35:363–370
Zarrinkalam MR, Schultz CG, Moore RJ (2009) Validation of the sheep as a large animal model for the study of vertebral osteoporosis. Eur Spine J 18:244–253
Zarrinkalam MR, Mulaibrahimovic A, Atkins GJ, Moore RJ (2012) Changes in osteocyte density correspond with changes in osteoblast and osteoclast activity in an osteoporotic sheep model. Osteoporos Int 23:1329–1336
Oheim R, Beil FT, Barvencik F, Egermann M, Amling M, Clarke IJ, Pogoda P (2012) Targeting the lateral but not the third ventricle induces bone loss in ewe: an experimental approach to generate an improved large animal model of osteoporosis. J Trauma Acute Care Surg 72:720–726
Bindl R, Oheim R, Pogoda P, Beil FT, Gruchenberg K, Reitmaier S, Wehner T, Calcia E, Radermacher P, Claes L, Amling M, Ignatius A (2013) Metaphyseal fracture healing in a sheep model of low turnover osteoporosis induced by hypothalamic-pituitary disconnection (HPD). J Orthop Res 31:1851–1857
Beil FT, Oheim R, Barvencik F, Hissnauer TN, Pestka JM, Ignatius A, Rueger JM, Schinke T, Clarke IJ, Amling M, Pogoda P (2012) Low turnover osteoporosis in sheep induced by hypothalamic-pituitary disconnection. J Orthop Res 30:1254–1262
Egermann M, Gerhardt C, Barth A, Maestroni GJ, Schneider E, Alini M (2011) Pinealectomy affects bone mineral density and structure–an experimental study in sheep. BMC Musculoskelet Disord 12:271
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Obuchowski NA, McClish DK (1997) Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med 16:1529–1542
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Jt Surg (American) 86-a:455–464
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17
Delling G (1975) Endokrine Osteopathien. Gustav Fischer Verlag, Stuttgart, Germany
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326
Merz WA (1967) Die Streckenmessung an gerichteten Strukturen im Mikroskop und ihre Anwendung zur Bestimmung von Oberflächen-Volumen-Relationen im Knochengewebe. Mikroskopie 22:132–142
Ma S, Goh EL, Jin A, Bhattacharya R, Boughton OR, Patel B, Karunaratne A, Vo NT, Atwood R, Cobb JP, Hansen U, Abel RL (2017) Long-term effects of bisphosphonate therapy: perforations, microcracks and mechanical properties. Sci Rep 7:43399
Vesterby A, Gundersen HJ, Melsen F (1989) Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone 10:7–13
Wainwright SA, Biggs WD, Currey JD, Gosline JM (1982) Mechanical design in organisms. Princeton University Press, Princeton
Mori R, Kodaka T, Soeta S, Sato J, Kakino J, Hamato S, Takaki H, Naito Y (2005) Preliminary study of histological comparison on the growth patterns of long-bone cortex in young calf Pig, and Sheep. J Vet Med Sci 67:1223–1229
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Adinoff AD, Hollister JR (1983) Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med 309:265–268
Kettler A, Liakos L, Haegele B, Wilke HJ (2007) Are the spines of calf, pig and sheep suitable models for pre-clinical implant tests? Eur Spine J 16:2186–2192
Potes JC, Reis J, Capela e Silva F, Relvas C, Cabrita AS, Simões JA (2008) The Sheep as an animal model in orthopaedic research. Exp Pathol Health Sci 2:29–32
Liebschner MA (2004) Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 25:1697–1714
Benneker LM, Krebs J, Boner V, Boger A, Hoerstrup S, Heini PF, Gisep A (2010) Cardiovascular changes after PMMA vertebroplasty in sheep: the effect of bone marrow removal using pulsed jet-lavage. Eur Spine J 19:1913–1920
Maenz S, Brinkmann O, Kunisch E, Horbert V, Gunnella F, Bischoff S, Schubert H, Sachse A, Xin L, Gunster J, Illerhaus B, Jandt KD, Bossert J, Kinne RW, Bungartz M (2017) Enhanced bone formation in sheep vertebral bodies after minimally invasive treatment with a novel PLGA fiber-reinforced brushite cement. Spine J 17:709–719
Bungartz M, Kunisch E, Maenz S, Horbert V, Xin L, Gunnella F, Mika J, Borowski J, Bischoff S, Schubert H, Sachse A, Illerhaus B, Gunster J, Bossert J, Jandt KD, Ploger F, Kinne RW, Brinkmann O (2017) GDF5 significantly augments the bone formation induced by an injectable PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J 17:1685–1698
Gunnella F, Kunisch E, Bungartz M, Maenz S, Horbert V, Xin L, Mika J, Borowski J, Bischoff S, Schubert H, Hortschansky P, Sachse A, Illerhaus B, Gunster J, Bossert J, Jandt KD, Ploger F, Kinne RW, Brinkmann O (2017) Low-dose BMP-2 is sufficient to enhance the bone formation induced by an injectable PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J 17:1699–1711
Gunnella F, Kunisch E, Maenz S, Horbert V, Xin L, Mika J, Borowski J, Bischoff S, Schubert H, Sachse A, Illerhaus B, Gunster J, Bossert J, Jandt KD, Ploger F, Kinne RW, Brinkmann O, Bungartz M (2018) The GDF5 mutant BB-1 enhances the bone formation induced by an injectable, poly(l-lactide-co-glycolide) acid (PLGA) fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J 18:357–369
Smit TH (2002) The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 11:137–144
Lorentzon M, Cummings SR (2015) Osteoporosis: the evolution of a diagnosis. J Int Med 277:650–661
Diez-Perez A, Güerri R, Nogues X, Cáceres E, Peña MJ, Mellibovsky L, Randall C, Bridges D, Weaver JC, Proctor A, Brimer D, Koester KJ, Ritchie RO, Hansma PK (2010) Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 25:1877–1885
Acknowledgements
We gratefully acknowledge the financial support by the Carl Zeiss Foundation (doctoral candidate scholarship to S.M.) and by the German Federal Ministry of Education and Research (BMBF FKZ 0316205C to J.B and K.D.J.; BMBF FKZ 035577D, 0316205B, and 13N12601 to R.W.K).
Author information
Authors and Affiliations
Contributions
SM designed the study and wrote the initial draft of the manuscript. Other authors have contributed to animal care, data collection and interpretation (OB, IH, CB, EK, VH, FG, AS, SB, HS, KDJ, JB). MB and RWK critically reviewed the manuscript. All authors contributed to analysis and interpretation of data, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no other relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Maenz, S., Brinkmann, O., Hasenbein, I. et al. The old sheep: a convenient and suitable model for senile osteopenia. J Bone Miner Metab 38, 620–630 (2020). https://doi.org/10.1007/s00774-020-01098-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01098-x