Abstract
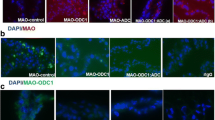
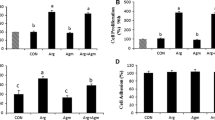
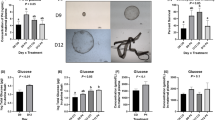
This study investigated the effect of agmatine (Agm) in proliferation of ovine trophecdoderm cells (oTr1) as well as the importance of the arginine decarboxylase (ADC) and agmatinase (AGMAT) alternative pathway for synthesis of polyamines in ovine conceptuses during the peri-implantation period of pregnancy. Morpholino antisense oligonucleotides (MAOs) were used to inhibit translation of mRNAs for ODC1 alone, AGMAT alone, and their combination. Rambouillet ewes (N = 50) were assigned randomly to the following treatments on Day 8 of pregnancy: MAO control (n = 10); MAO-ODC1 (n = 8); MAO-ADC (n = 6); MAO-ODC1:MAO-ADC (n = 9); or MAO-ODC1:MAO-AGMAT (n = 9). Ewes were ovario-hysterectomized on Day 16 of pregnancy to obtain uterine flushings, uterine endometrium, and conceptus tissues. Inhibition of translation of both ODC1 and AGMAT resulted in 22% of ewes having morphologically and functionally normal (elongated and healthy) conceptuses designated MAO-ODC1:MAO-AGMAT (A). But, 78% of the MAO-ODC1:MAO-AGMAT ewes had morphologically and functionally abnormal (not elongated and fragmented) conceptuses designated MAO-ODC1:MAO-AGMAT (B). The pregnancy rate was less (22%; P < 0.05) for MAO-ODC1:MAO-AGMAT ewes than for MAO-control (80%), MAO-ODC1 (75%), MAO-ADC (84%), and MAO-ODC1:MAO-ADC (44%) ewes. Moreover, inhibition of translational of both ODC1 and AGMAT mRNAs increased expression of ADC, SLC22A1, SLC22A2, and SLC22A3 mRNAs, as well as abundances of agmatine, putrescine, spermindine, and spermine in conceptus tissue. However, MAO-ODC1:AGMAT(B) ewes had greater abundances of agmatine, putrescine, and spermidine and reduced amounts of spermine in uterine flushes. Thus, in vivo knockdown of translation of ODC1 and AGMAT mRNAs increased expression of genes for the synthesis and transport of polyamines in ovine conceptuses during the peri-implantation period of pregnancy.











Similar content being viewed by others
Abbreviations
- ADC :
-
Arginine decarboxylase
- AGMAT:
-
Agmatinase
- Arg:
-
l-arginine
- Agm:
-
Agmatine
- OAZ:
-
Antienzyme
- MAO:
-
Morpholino
- IFNT:
-
Interferon tau
- IGF2:
-
Insulin growth factor type 2
- NO:
-
Nitric oxide
- ODC1 :
-
Ornithine decarboxylase
- SLC22A1:
-
Organic cationic transporter type 1
- SLC22A2:
-
Organic cationic transporter type 2
- SLC22A3:
-
Organic cationic transporter type 3
- SLC7A1:
-
Solute carrier family 7 member 1
References
Abdulhussein AA, Wallace HM (2014) Polyamines and membrane transporters. Amino acids 46(3):655–660
Arqués O, Chicote I, Tenbaum et al (2012) Standardized relative quantification of immunofluorescence tissue staining. Protoc Exch 10. https://doi.org/10.1038/protex.2012.008
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP (2008) The impact of microRNAs on protein output. Nature 455:64–71
Bazer FW, Spencer TE, Ott TL (1997) Interferon tau: a novel pregnancy recognition signal. Am J Reprod Immunol 37:412–420
Bazer FW, Spencer TE, Johnson GA et al (2010a) Uterine receptivity to implantation of blastocysts in mammals. Front Biosci (Schol Ed) 3:745–767
Bazer FW, Wu G, Spencer TE et al (2010b) Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 16:135–152
Bazer FW, Wu G, Johnson GA et al (2011) Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol Reprod 85:1094–1107
Bazer FW, Kim J, Song G et al (2012) Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Ann N Y Acad Sci 1271:88–96
Bazer FW, Wu G, Johnson GA et al (2014) Environmental factors affecting pregnancy: endocrine disrupters, nutrients and metabolic pathways. Mol Cell Endocrinol 398:53–68
Dai Z, Wu Z, Wang Jia S, Bazer FW, Wu G (2014) Analysis of polyamines in biological samples by HPLC involving pre-column derivatization with O-phthalaldehyde and N-acetyl-L-cysteine. Amino Acids 46:1557–1564
DiFederico E, Genbacev O, Fisher SJ (1999) Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 155:293–301
Gao H, Wu G, Spencer TE et al (2009a) Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80:86–93
Gao H, Wu G, Spencer TE et al (2009b) Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod 80:602–609
Gray CA, Burghardt RC, Johnson GA et al (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124:289–300
Gray CA, Abbey CA, Beremand PD et al (2006) Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod 74:383–394
Hilal T, Spahn CM (2015) Ribosome rescue and protein quality control in concert. Mol Cell 57:389–390
Irwin JC, Suen LF, Martina NA et al (1999) Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum reprod 14(2):90–98
Jayme D, Watanabe T, Shimada T (1997) Basal medium development for serum-free culture: a historical perspective. Cytotechnology 23:95–101
Kim J, Wende AR, Sena S et al (2008) Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol 22:2531–2543
Kong X, Wang X, Yin Y et al (2014) Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod 91(5):106–111
Kwon H, Wu G, Bazer FW et al (2003) Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod 69:1626–1634
Lefèvre PL, Palin MF, Murphy BD (2011) Polyamines on the reproductive landscape. Endocr Rev 32(5):694–712
Lenis YY, Wang X, Tang W et al (2016) Effects of agmatine on secretion of interferon tau and catecholamines and expression of genes related to production of polyamines by ovine trophectoderm cells. Amino Acids 48(10):2389–2399
LeRoith D, Werner H, Beitner-Johnson D et al (1995) Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 16(2):143–163
Moinard C, Cynober L, de Bandt JP (2005) Polyamines: metabolism and implications in human diseases. Clin Nutr 24(2):184–197
Molderings GJ, Haenisch B (2012) Agmatine (decarboxylated l-arginine): physiological role and therapeutic potential. Pharmacol Ther 133:351–365
Piletz JE, Aricioglu F, Cheng JT et al (2013) Agmatine: clinical applications after 100 years in translation. Drug Discov Today 18(17):880–893
Raspotnig G, Fauler G, Jantscher A (1999) Colorimetric determination of cell numbers by janus green staining. Anal Biochem 275(1):74–83
Rechler MM, Nissley SP (1985) The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol 47(1):425–442
Rezaei R, Knabe A, Tekwe D et al (2013) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44(3):911–923
Sala-Rabanal M, Li DC, Dake GR et al (2013) Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm 10(4):1450–1458
Sastre M, Regunathan S, Galea E et al (1996) Agmatinase activity in rat brain: a metabolic pathway for the degradation of agmatine. J Neurochem 67(4):1761–1765
Satterfield MC, Hayashi K, Song G et al (2008) Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 79(6):1226–1236
Sekowska A, Bertin P, Danchin A (1998) Characterization of polyamine synthesis pathway in Bacillus subtilis 168. Mol Microbiol 29(3):851–858
Spencer TE, Bazer FW (2004) Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci 82:4–13
Spencer TE, Gray A, Johnson GA et al (1999) Effects of recombinant ovine interferon tau, placental lactogen, and growth hormone on the ovine uterus. Biol Reprod 61:1409–1418
Spencer TE, Johnson GA, Bazer FW et al (2004) Implantation mechanisms: insights from the sheep. Reproduction 128:657–668
Spencer TE, Johnson GA, Bazer FW et al (2006) Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev 19:65–78
Spencer TE, Forde N, Lonergan P (2017) Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod Fertil Dev 29:84–100
Wang X, Wei Y, Dunlap KA et al (2014) Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90:84
Wang X, Burghardt RC, Romero JJ et al (2015) Functional roles of arginine during the peri-implantation period of pregnancy. III. Arginine stimulates proliferation and interferon tau production by ovine trophectoderm cells via nitric oxide and polyamine-TSC2-MTOR signaling pathways. Biol Reprod 92:75
Wolf C, Brüss M, Hänisch B et al (2007) Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol 71(1):276–283
Wu G, Meininger CJ (2000) Arginine nutrition and cardiovascular function. J Nutr 130:2626–2629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Bazer FW, Cudd TA et al (2004) Maternal nutrition and fetal development. J Nutr 134:2169–2172
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Satterfield MC et al (2013) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Zhao YC, Chi YJ, Yu YS et al (2008) Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 149(5):2325–2332
Acknowledgements
Funding from the Sustainability Strategy 2013–2014, from CODI University of Antioquia (UdeA), Medellín, Colombia Scholarship “Becas Doctorado UdeA 2014” was used to support YYL, a PhD student in Veterinary Science, Faculty of Agrarian Science, Antioquia University). Funding for the research and related activities was from the Agriculture and Food Research Initiative Competitive Grants (2016-67015-24958 to FWB and 2015-67015-23276 to GW) from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Additional information
Handling Editor: F. Erdmann.
Rights and permissions
About this article
Cite this article
Lenis, Y.Y., Elmetwally, M.A., Tang, W. et al. Functional roles of agmatinase during the peri-implantation period of pregnancy in sheep. Amino Acids 50, 293–308 (2018). https://doi.org/10.1007/s00726-017-2515-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2515-1