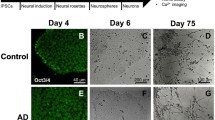
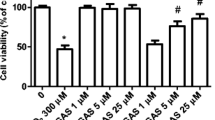
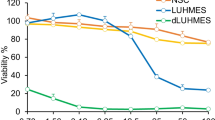
Abstract
Oxidative stress has been suggested to play an important role in the pathogenesis of various neurodegenerative diseases including Alzheimer’s disease (AD). Hydrogen peroxide (H2O2), one of the main reactive oxygen species, is converted into the highly toxic ·OH radical in the presence of redox-active transition metals, which then oxidises nucleic acids, lipids and proteins, leading to neurodegeneration and cell death. There is an urgent need to gain more knowledge about relevant therapeutic targets to combat oxidative stress and it neurotoxic effects, and how this knowledge can be utilized to develop novel neuroprotective therapies for AD. One way to identify new mechanisms combating oxidative stress was via the creation of H2O2-resistant cell lines and identification of the mechanisms responsible for their resistance. However, in most cases catalase overexpression or increased glutathione content was identified as the primary mode of H2O2 resistance in these cell lines. In this study, we have generated six different resistant neuronal cell lines or populations (from the same original murine Neuro2a neuroblastoma line) by exposing cells to increasing concentrations of H2O2 and performing continuous selection for survivors over a period of several months, which appear to have acquired H2O2 resistance based on other, novel mechanisms. These six populations showed a significant, but differential resistance against H2O2 when compared with the parental cell line. Using combinations of catalase-, glutathione synthesis- and glutathione peroxidase-inhibitors it was shown that the increased resistance of Neuro2a-HR cells is not solely based on an increased activity of catalase or the glutathione system, suggesting that their resistance might be based on yet unknown, novel defence mechanisms.


Similar content being viewed by others
References
Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, Markesbery WR (1998) The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. J Mol Neurosci 11(2):151–164. doi:10.1385/JMN:11:2:151
Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR (2001) Protein oxidation in the brain in Alzheimer’s disease. Neuroscience 103(2):373–383
Behl C (1997) Amyloid beta-protein toxicity and oxidative stress in Alzheimer’s disease. Cell Tissue Res 290(3):471–480
Butterfield DA, Kanski J (2001) Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev 122(9):945–962
Cantoni O, Guidarelli A, Sestili P, Mannello F, Gazzanelli G, Cattabeni F (1993) Development and characterization of hydrogen peroxide-resistant Chinese hamster ovary cell variants—I. Relationship between catalase activity and the induction/stability of the oxidant-resistant phenotype. Biochem Pharmacol 45(11):2251–2257
Dringen R, Hamprecht B (1997) Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759(1):67–75
Dringen R, Kussmaul L, Hamprecht B (1998) Rapid clearance of tertiary butyl hydroperoxide by cultured astroglial cells via oxidation of glutathione. Glia 23(2):139–145
Gay C, Collins J, Gebicki JM (1999) Determination of hydroperoxides by the ferric-xylenol orange method. Redox Rep 4(6):327–328
Giblin FJ, Reddan JR, Schrimscher L, Dziedzic DC, Reddy VN (1990) The relative roles of the glutathione redox cycle and catalase in the detoxification of H2O2 by cultured rabbit lens epithelial cells. Exp Eye Res 50(6):795–804
Gsell W, Conrad R, Hickethier M, Sofic E, Frolich L, Wichart I, Jellinger K, Moll G, Ransmayr G, Beckmann H et al (1995) Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J Neurochem 64(3):1216–1223
John O’Brien IW (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267(17):5421–5426
Kussmaul L, Hamprecht B, Dringen R (1999) The detoxification of cumene hydroperoxide by the glutathione system of cultured astroglial cells hinges on hexose availability for the regeneration of NADPH. J Neurochem 73(3):1246–1253
Liu H, Wang H, Shenvi S, Hagen TM, Liu RM (2004) Glutathione metabolism during aging and in Alzheimer disease. Ann N Y Acad Sci 1019:346–349
Lovell MA, Ehmann WD, Butler SM, Markesbery WR (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 45(8):1594–1601
Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML (1998) Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol 150(1):40–44
Münch G, Schinzel R, Loske C, Wong A, Durany N, Li JJ, Vlassara H, Smith MA, Perry G, Riederer P (1998) Alzheimer’s disease—synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm 105(4–5):439–461
Park YM, Anderson RL, Spitz DR, Hahn GM (1992) Hypoxia and resistance to hydrogen peroxide confer resistance to tumor necrosis factor in murine L929 cells. Radiat Res 131(2):162–168
Ramakrishnan A, Sandmaier BM (2010) Optimizing reduced-intensity conditioning regimens for myeloproliferative neoplasms. Expert Rev Hematol 3(1):23–33. doi:10.1586/ehm.09.73
Retz W, Gsell W, Munch G, Rosler M, Riederer P (1998) Free radicals in Alzheimer’s disease. J Neural Transm Suppl 54:221–236
Schafer M, Goodenough S, Moosmann B, Behl C (2004) Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res 1005(1–2):84–89. doi:10.1016/j.brainres.2004.01.037
Spitz DR, Li GC, McCormick ML, Sun Y, Oberley LW (1988) The isolation and partial characterization of stable H2O2-resistant variants of Chinese hamster fibroblasts. Basic Life Sci 49:549–552
Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA (2006) Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging 27(11):1564–1576. doi:10.1016/j.neurobiolaging.2005.09.021
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA 91(11):4766–4770
Acknowledgments
We thank Merz Pharmaceutical GmbH for their financial support for the conduct of the research. The sponsor did not participate in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maczurek, A.E., Wild, R., Laurenti, D. et al. Generation of hydrogen peroxide-resistant murine neuroblastoma cells: a target discovery platform for novel neuroprotective genes. J Neural Transm 120, 1171–1178 (2013). https://doi.org/10.1007/s00702-013-0995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-0995-z