Abstract
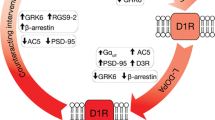
Continuous dopaminergic treatment is considered to prevent or delay the occurrence of dyskinesia in patients with Parkinson’s disease (PD). Rotigotine is a non-ergolinic D3 > D2 > D1 dopamine-receptor agonist for the treatment of PD using a transdermal delivery system providing stable plasma levels. We aimed to investigate the differential influence on gene expression of pulsatile l-DOPA or rotigotine versus a continuous rotigotine treatment. The gene expression profile within the nigro-striatal system of unilateral 6-hydroxydopamine-lesioned rats was assessed in order to differentiate potential changes in gene expression following the various treatment using Affymetrix microarrays and quantitative RT-PCR. The expression of 15 genes in the substantia nigra and of 11 genes in the striatum was altered under pulsatile treatments inducing dyskinetic motor response, but was unchanged under continuous rotigotine treatment that did not cause dyskinetic motor response. The route of administration of a dopaminergic drug is important for the induction or prevention of motor abnormalities and adaptive gene expressions. The decline of neurotrophin-3 expression under pulsatile administration was considered of particular importance.
Similar content being viewed by others
References
Alam M, Schmidt WJ (2004) L-DOPA reverses the hyperkinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res 153:439–446
Apfel SC (1999) Neurotrophic factors in peripheral neuropathies: therapeutic implications. Brain Pathol 9:393–413
Baldwin CM, Keating GM (2007) Rotigotine transdermal patch: a review of its use in the management of Parkinson’s disease. CNS Drugs 21:1039–1055
Brotchie JM (2005) Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord 20:919–931
Cenci MA (2002) Transcription factors involved in the pathogenesis of l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids 23:105–109
Cenci MA (2007) Dopamine dysregulation of movement control in l-DOPA-induced dyskinesia. Trends Neurosci 30:236–243
Cenci MA, Lee CS, Bjorklund A (1998) l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706
Chase TN (2004) Striatal plasticity and extrapyramidal motor dysfunction. Parkinsonism Relat Disord 10:305–313
Elshoff JP, Cawello W, Braun M, Horstmann R (2006) Stable rotigotine plasma concentrations over 24 hours in patients with early-stage Parkinson’s disease after once-daily transdermal administration of rotigotine (Neupro (R)). Lippincott Williams & Wilkins, Baltimore, pp 293
Giladi N, Boroojerdi B, Korczyn AD, Burn DJ, Clarke CE, Schapira AH (2007) Rotigotine transdermal patch in early Parkinson’s disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 22:2398–2404
Grünblatt E, Hoyer S, Riederer P (2004a) Gene expression profile in streptozotocin rat model for sporadic Alzheimer’s disease. J Neural Transm 111:367–386
Grünblatt E, Mandel S, Jacob-Hirsch J et al (2004b) Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm 111:1543–1573
Gu S, Huang H, Bi J, Yao Y, Wen T (2009) Combined treatment of neurotrophin-3 gene and neural stem cells is ameliorative to behavior recovery of Parkinson’s disease rat model. Brain Res 1257:1–9
Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E (2007) Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Dis 26:452–463
Jacob CP, Koutsilieri E, Bartl J et al (2007) Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis 11:97–116
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20:(Suppl 11):S11–S16
Jenner P (2008) Molecular mechanisms of l-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677
Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA (2004) Transcriptome analysis in a rat model of l-DOPA-induced dyskinesia. Neurobiol Dis 17:219–236
Li Z, Hu Y, Zhu Q, Zhu J (2008) Neurotrophin-3 reduces apoptosis induced by 6-OHDA in PC12 cells through Akt signaling pathway. Int J Dev Neurosci 26:635–640
Maeda T, Nagata K, Yoshida Y, Kannari K (2005) Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l-DOPA. Brain Res 1046:230–233
Metz GA, Tse A, Ballermann M, Smith LK, Fouad K (2005) The unilateral 6-OHDA rat model of Parkinson’s disease revisited: an electromyographic and behavioural analysis. Eur J Neurosci 22:735–744
Morelli M, Fenu S, Cozzolino A, Di Chiara G (1991) Positive and negative interactions in the behavioural expression of D1 and D2 receptor stimulation in a model of Parkinsonism: role of priming. Neuroscience 42:41–48
Nutt JG (2007) Continuous dopaminergic stimulation: is it the answer to the motor complications of Levodopa? Mov Disord 22:1–9
Paxinos G, Watson C (1986) The rat brain in sterotaxic coordinates. Academic Press, London
Schmidt WJ, Lebsanft H, Heindl M, Gerlach M, Grünblatt E, Riederer P, Mayerhofer A, Scheller DK (2008) Continuous versus pulsatile administration of rotigotine in 6-OHDA-lesioned rats: contralateral rotations and abnormal involuntary movements. J Neural Transm 115:1385–1392
Sgambato-Faure V, Buggia V, Gilbert F, Levesque D, Benabid AL, Berger F (2005) Coordinated and spatial upregulation of arc in striatonigral neurons correlates with l-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol 64:936–947
Steiger M (2008) Constant dopaminergic stimulation by transdermal delivery of dopaminergic drugs: a new treatment paradigm in Parkinson’s disease. Eur J Neurol 15:6–15
Stockwell KA, Scheller DK, Smith LA, Rose S, Iravani MM, Jackson MJ, Jenner P (2010) Continuous rotigotine administration reduces dyskinesia resulting from pulsatile treatment with rotigotine or l-DOPA in MPTP-treated common marmosets. Exp Neurol 221:79–85
Valastro B, Dekundy A, Krogh M, Lundblad M, James P, Danysz W, Quack G, Cenci MA (2007) Proteomic analysis of striatal proteins in the rat model of l-DOPA-induced dyskinesia. J Neurochem 102:1395–1409
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, RESEARCH0034
von Bohlen und Halbach O, Minichiello L, Unsicker K (2005) Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. FASEB J 19:1740–1742
Acknowledgments
The authors thank for the help in the analysis of the Gene Chip Affymetrix arrays by Dr. Susanne Kneitz. And a special thank for devoted technical work provided by Ms. Miryame Hofmann. The study was sponsored by SCHWARZ BIOSCIENCES, Alfred-Nobel Strasse 10, 40789, Monheim, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grünblatt, E., Schmidt, W.J., Scheller, D.K.A. et al. Transcriptional alterations under continuous or pulsatile dopaminergic treatment in dyskinetic rats. J Neural Transm 118, 1717–1725 (2011). https://doi.org/10.1007/s00702-010-0552-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0552-y