Abstract
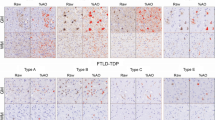
Studies suggest that frontotemporal lobar degeneration with transactive response DNA-binding protein of 43 kDa (TDP-43) proteinopathy (FTLD-TDP) is heterogeneous with division into four or five subtypes. To determine the degree of heterogeneity and the validity of the subtypes, we studied neuropathological variation within the frontal and temporal lobes of 94 cases of FTLD-TDP using quantitative estimates of density and principal components analysis (PCA). A PCA based on the density of TDP-43 immunoreactive neuronal cytoplasmic inclusions, oligodendroglial inclusions, neuronal intranuclear inclusions, and dystrophic neurites, surviving neurons, enlarged neurons, and vacuolation suggested that cases were not segregated into distinct subtypes. Variation in the density of the vacuoles was the greatest source of variation between cases. A PCA based on TDP-43 pathology alone suggested that cases of FTLD-TDP with progranulin (GRN) mutation segregated to some degree. The pathological phenotype of all four subtypes overlapped but subtypes 1 and 4 were the most distinctive. Cases with coexisting motor neuron disease (MND) or hippocampal sclerosis (HS) also appeared to segregate to some extent. We suggest: (1) pathological variation in FTLD-TDP is best described as a ‘continuum’ without clearly distinct subtypes, (2) vacuolation was the single greatest source of variation and reflects the ‘stage’ of the disease, and (3) within the FTLD-TDP ‘continuum’ cases with GRN mutation and with coexisting MND or HS may have a more distinctive pathology.







Similar content being viewed by others
References
Armstrong RA (1996) Correlations between the morphology of diffuse and primitive β-amyloid (Aβ) deposits and the frequency of associated cells in Down’s syndrome. Neuropathol Appl Neurobiol 22:527–530
Armstrong RA (2003) Quantifying the pathology of neurodegenerative disorders: quantitative measurements, sampling strategies and data analysis. Histopathology 42:521–529
Armstrong RA, Nochlin D, Bird TD (2000) Neuropathological heterogeneity in Alzheimer’s disease: a study of 80 cases using principal components analysis. Neuropathology 20:31–37
Armstrong RA, Lantos PL, Cairns NJ (2001) Spatial correlations between the vacuolation, prion protein deposits, and surviving neurons in the cerebral cortex in sporadic Creutzfeldt–Jakob disease. Neuropathology 21:266–271
Armstrong RA, Ironside J, Lantos PL, Cairns NJ (2009) A quantitative study of the pathological changes in the cerebellum of 15 cases of variant Creutzfeldt–Jakob disease. Neuropathol Appl Neurobiol 35:36–45
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ (2007) Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer Dis Assoc Disord 21:1–7
Bland JM, Altman DG (1986) Statistical method for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Braak H, Braak E, Bohl J (1993) Staging of Alzheimer-related cortical destruction. Eur Neurol 33:403–408
Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IRA, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Lee VMY, Trojanowski JQ (2004) Alpha-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol 164:2153–2161
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Steiber A, Xu Y, Forman MS, Trojanowski JQ, Lee VMY, Mackenzie IRA (2007a) TDP-43 familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA (2007b) Neuropathologic diagnostic and nosological criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Cruts M, Gijselink I, van der ZJ, Engelborgs S, Wils H, Pirici D, Radamakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De pooter T, Mattheijssens M, van den BM, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Davidson Y, Kelley T, Mackenzie IRA, Pickering Brown S, Du Plessis D, Neary D, Snowden JS, Mann DMA (2007) Ubiquinated pathological lesions in frontotemporal lobar degeneration contain TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Davion S, Johnson N, Weintraub S, Mesulam MM, Engberg A, Mishra M, Baker M, Adamson J, Hutton M, Rademakers R, Bigio EH (2007) Clinicopathologic correlations in PGRN mutations. Neurology 69:1113–1121
Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestrvik A, Smith TN, Tu PH, Watts GDJ, Markesbery WR, Smith CD, Kimonis VE (2006) Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol 65:571–581
Hatanpaa KJ, Bigio EH, Cairns NJ, Womack KB, Weintraub S, Morris JC, Foong C, Xiao GH, Hladik C, Mantanona TY, White CL (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a Midwest-Southwest Consortium for FTLD-U study. J Neuropathol Exp Neurol 67:271–279
Josephs KA (2008) Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol 64:4–14
Josephs KA, Knopman DS, Whitwell JL, Boeve BF, Parisi JE, Petersen RC, Dickson DW (2005) Survival in the two variants of tau negative FTLD: FTLD-U versus FTLD-MND. Neurology 65:645–647
Josephs KA, Whitwell JL, Jack CR, Parisi JE, Dickson DW (2006) Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol 63:1632–1638
Kersaitis C, Holliday GM, Xuereb JH, Pamphlett R, Bak TH, Hodges JR, Kril JJ (2006) Ubiquitin-positive inclusions and progression of pathology in frontotemporal dementia and motor neurone disease identifies a group with mainly early pathology. Neuropathol Appl Neurobiol 32:83–91
Kovari E, Gold G, Giannakopoulos P, Bouras C (2004) Cortical ubiquitin positive inclusions in frontotemporal dementia without motor neuron disease: a quantitative immunocytochemical study. Acta Neuropathol 108:207–212
Luty AA, Kwok JBJ, Thompson EM, Blumsbergs P, Brooks WS, Loy CT, Dobson-Stone C, Panegyres PK, Hecker J, Nicholson GA, Halliday GM, Schofield PR (2008) Pedigree with frontotemporal lobar degeneration-motor neuron disease and Tar DNA binding protein-43 positive neuropathology: genetic linkage to chromosome 9. BMC Neurol 8:32
Mackenzie IRA, Baker M, Pickering-Brown S, Hsinng GYR, Lindholm C, Dwosh E, Cannon A, Rademakers R, Hutton M, Feldman HH (2006a) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA (2006b) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549
Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJM, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson D, Trojanowski JQ, Mann DMA (2009) Nomenclature for neuropathologic subtypes of frontotemporal degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Mukherjee O, Pastor P, Cairns NJ, Chakraaverty S, Kauwe JSK, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Grinberg LT, Liscic RM, Armendariz J, Morris JC, Goate AM (2006) HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 60:314–322
Neumann M, Igaz LM, Kwong LK, Nakashima-Yasuda H, Kolb SJ, Dreyfuss G, Kretzschmar HA, Trojanowski JQ, Lee VMY (2007) Absence of heterogeneous nuclear riboproteins and survival neuron protein (TDP-43) positive inclusions in frontotemporal lobar degeneration. Acta Neuropathol 113:543–548
Pirici D, Vandenberghe R, Rademakers R, Dermant B, Cruts M, Vennekens K, Cuijt I, Lubke U, Centerick C, Martin JJ, Van Broeckhoven C, Kumar-Singh S (2006) Characterization of ubiquinated intraneuronal inclusions in a novel Belgian frontotemporal lobar degeneration family. J Neuropathol Exp Neurol 65:289–301
Rademakers R, Hutton M (2007) The genetics of frontotemporal lobar degeneration. Curr Neurol Neurosci Rep 7:434–442
Ritchie K, Touchon J (1992) Heterogeneity in senile dementia of the Alzheimer type: individual differences, progressive deterioration or clinical subtypes? J Clin Epidemiol 45:1391–1398
Rollinson S, Rizzu P, Sikkink S, Baker M, Halliwell N, Snowden J, Traynor BJ, Ruano D, Cairns N, Rohrer JD, Mead S, Collinge J, Rossor M, Akay E, Gueireiro R, Rademakers R, Morrison KE, Pastor P, Alonso E, Martinez-Lage P, Graff-Radford N, Neary D, Henlink P, Mann DMA, Van Swieten J, Pickering-Brown SM (2009) Ubiquitin associated protein 1 is a risk factor for frontotemporal lobar degeneration. Neurobiol Aging 30:656–665
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immonohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Snowden J, Neary D, Mann D (2007) Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 114:31–38
Tolnay M, Probst A (2002) Frontotemporal lobar degeneration—tau as a pied piper? Neurogenetics 4:63–75
Van Deerlin VM, Wood EM, Moore P, Yuan W, Forman MS, Clark CM, Neumann M, Kwong LK, Trojanoswki JQ, Lee VMY, Grossman M (2007) Clinical, genetic and pathologic characteristics of patients with frontotemporal dementia and progranulin mutation. Arch Neurol 64:1148–1153
Van der Zee J, Gyselinck I, Pirici D, Kumar-Singh S, Cruts M, van Broeckhoven C (2007) Frontotemporal lobar degeneration with ubiquitin-positive inclusions: a molecular genetic update. Neurodegener Dis 4:227–235
Whitwell JL, Jack CR, Serijeni ML, Josephs KA (2006) Patterns of atrophy in pathologically confirmed FTLD with or without motor neuron degeneration. Neurology 66:102–104
Woulfe J, Kertesz A, Munoz DG (2001) Frontotemporal dementia with ubiquinated cytoplasmic and intranuclear inclusions. Acta Neuropathol 102:94–102
Yaguchi M, Fujita Y, Amari M, Takatama M, Al-Sarraj S, Leigh PN, Okamoto K (2004) Morphological differences of intraneural ubiquitin positive inclusions in the dentate gyrus and parahippocampal gyrus of motor neuron disease with dementia. Neuropathology 24:296–301
Acknowledgements
We thank clinical, genetic, pathology, and technical staff of the participating centres and especially C. Kaminski, Centre for Neurodegenerative Disease Research, University of Pennsylvania for making information and tissue samples available for this study and we thank the families of patients whose generosity made this research possible. Support for this work was provided by grants from the NIH (National Institute on Aging): P50-AG05681, P01-AG03991, U01-AG16976, P30-AG13854, and P30-NS057105 to N.J. Cairns, P50-AG16573 and P50-AG000658 to E. Head, P50-AG05133 to Pittsburgh, Alzheimer’s Disease Research Center, and P50AG008671 to A.P. Lieberman, the Hope Center for Neurological Disorders to N.J. Cairns, and the Charles Knight Fund to N.J. Cairns.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, R.A., Ellis, W., Hamilton, R.L. et al. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm 117, 227–239 (2010). https://doi.org/10.1007/s00702-009-0350-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0350-6