Abstract
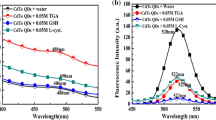
We have synthesized water dispersible CdTe quantum dots (QDs) in different sizes and with various capping reagents, and have studied the effects of their size on the sensitivity and selectivity in the fluorometric determination of metal ions, particularly of silver(I). It is found that an increase in the particle size of homocysteine-capped CdTe QDs from 1.7 nm to 3.3 nm and to 3.7 nm enhances both the sensitivity and selectivity of the determination of Ag(I) to give an ultimate limit of detection as low as 8.3 nM. This effect can partially be explained by the better passivation of surface traps on smaller sized QDs via adsorption of Ag(I), thereby decreasing the apparent detection efficiency. In addition, the presence of CdS in the CdTe QDs is likely to play a role. The study demonstrates that an improvement in sensing performance is accomplished by using QDs of fine-tuned particle sizes. Such effects are likely also to occur with other QD-based optical probes.

Improvement of sensing performance by the use of quantum dots based fluorescent probes can be achieved through tuning their particle sizes and should be considered as an important factor in developing relevant probes.




Similar content being viewed by others
References
Aragay G, Pons J, Merkoci A (2011) Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111(5):3433–3458. doi:10.1021/cr100383r
Chan J, Dodani SC, Chang CJ (2012) Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat Chem 4(12):973–984. doi:10.1038/nchem.1500
Cheng X, Li S, Jia H, Zhong A, Zhong C, Feng J, Qin J, Li J (2012) Fluorescent and colorimetric probes for mercury(II): tunable structures of electron donor and p-conjugated bridge. Chem Eur J 18(6):1691–1699. doi:10.1002/chem.201102376
Li DH, Shen JS, Chen N, Ruan YB, Jiang YB (2011) A ratiometric luminescent sensing of Ag+ ion via in situ formation of coordination. Chem Commun 47(20):5900–5902. doi:10.1039/c0cc05519k
Yang RH, Chan WH, Lee AWM, Xia PF, Zhang HK, Li KA (2003) A ratiometric fluorescent sensor for Ag + with high selectivity and sensitivity. J Am Chem Soc 125(10):2884–2885. doi:10.1021/ja029253d
Jang S, Thirupathi P, Neupane LN, Seong J, Lee H, Lee WI, Lee KH (2012) Highly sensitive ratiometric fluorescent chemosensor for silver Ion and silver nanoparticles in aqueous solution. Org Lett 14(18):4746–4749. doi:10.1021/ol301991h
Merian E (1991) Metals and their compounds in the environment: occurrence. Analysis and Biological Relevance, VCH, New York
Poznyak SK, Osipovich NP, Shavel A, Talapin DV, Gao MY, Eychmuller A, Gaponik N (2005) Size-dependent electrochemical behavior of thiol-capped CdTe nanocrystals in aqueous solution. J Phys Chem B 109(3):1094–1100. doi:10.1021/jp0460801
Zhang J, Zhang X, Zhang JY (2009) Size-dependent time-resolved photoluminescence of colloidal CdSe nanocrystals. J Phys Chem C 113(22):9512–9515. doi:10.1021/jp9026354
Masumoto Y, Sonobe K (1997) Size-dependent energy levels of CdTe quantum dots. Phys Rev B 56(15):9734–9737. doi:10.1103/PhysRevB.56.9734
Qian H, Li L, Ren J (2005) One-step and rapid synthesis of high quality alloyed quantum dots (CdSe–CdS) in aqueous phase by microwave irradiation with controllable temperature. Mater Res Bull 40(10):1726–1736. doi:10.1016/j.materresbull.2005.05.022
Ma Q, Su X (2011) Recent advances and applications in QDs-based sensors. Analyst 136(23):4883–4893. doi:10.1039/c1an15741h
Ali EM, Zheng Y, Yu HH, Ying JY (2007) Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal Chem 79(24):9452–9458. doi:10.1021/ac071074x
Gan TT, Zhang YJ, Zhao NJ, Xiao X, Yin GF, Yu SH, Wang HB, Duan JB, Shi CY, Liu WQ (2012) Hydrothermal synthetic mercaptopropionic acid stabled CdTe quantum dots as fluorescent probes for detection of Ag+. Spectrochim Acta A 99:62–68. doi:10.1016/j.saa.2012.09.005
Xia YS, Zhu CQ (2008) Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 75(1):215–221. doi:10.1016/j.talanta.2007.11.008
Zhu X, Zhao Z, Chi X, Gao J (2013) Facile, sensitive, and ratiometric detection of mercuric ions using GSH-capped semiconductor quantum dots. Analyst 138(11):3230–3237. doi:10.1039/c3an00011g
Zhang K, Yu Y, Sun S (2013) Facile synthesis l-cysteine capped CdS:Eu quantum dots and their Hg2+ sensitive properties. Appl Surf Sci 276:333–339. doi:10.1016/j.apsusc.2013.03.093
Mahmoud WE (2012) Functionalized ME-capped CdSe quantum dots based luminescence probe for detection of Ba2+ ions. Sens Actuat B-chem 164(1):76–81. doi:10.1016/j.snb.2012.01.073
Pei J, Zhu H, Wang X, Zhang H, Yang X (2012) Synthesis of cysteamine-coated CdTe quantum dots and its application in mercury (II) detection. Anal Chim Acta 757:63–68. doi:10.1016/j.aca.2012.10.037
Duan J, Jiang X, Ni S, Yang M, Zhan J (2011) Facile synthesis of N-acetyl-L-cysteine capped ZnS quantum dots as an eco-friendly fluorescence sensor for Hg2+. Talanta 85(4):1738–1743. doi:10.1016/j.talanta.2011.06.071
Dong YP, Huang L, Tong BH, Shi MJ, Zhang WB, Zhang QF (2012) Enhancing and inhibiting effects of benzenediols on chemiluminescence of a novel cyclometallated iridium(III) complex. Luminescence 27(4):262–267. doi:10.1002/bio.1343
Laferriere M, Galian RE, Maurel V, Scaiano JC (2006) Non-linear effects in the quenching of fluorescent quantum dolts by nitroxyl free radicals. Chem Commun 42(3):257–259. doi:10.1039/b511515a
Xia YS, Cao C, Zhu CQ (2008) Two distinct photoluminescence responses of CdTe quantum dots to Ag (I). J Lumin 128(1):166–172. doi:10.1016/j.jlumin.2007.07.007
Xia Y, Zhang T, Diao X, Zhu C (2007) Measurable emission color change: Size-dependent reversible fluorescence quenching of CdTe quantum dots by molecular oxygen. Chem Lett 36(2):242–243. doi:10.1246/cl.2007.242
Zou l, Gu Z, Zhang N, Zhang Y, Fang Z, Zhu W, Zhong X (2008) Ultrafast synthesis of highly luminescent green- to near infrared-emitting CdTe nanocrystals in aqueous phase. J Mater Chem 18(24):2807–2815. doi:10.1039/b801418c
Qu L, Yu WW, Peng X (2004) In Situ Observation of the Nucleation and Growth of CdSe Nanocrystals. Nano Lett 4(3):465–469. doi:10.1021/nl035211r
Mei YL, Wang HS, Li YF, Pan ZY, Jia WL (2010) Electochemiluminescence of CdTe/CdS Quantum Dots with Triproprylamine as Coreactant in Aqueous Solution at a Lower Potential and Its Application for Highly Sensitive and Selective Detection of Cu2+. Electroanal 22(2):155–160. doi:10.1002/elan.200904685
Zheng Y, Gao S, Ying JY (2007) Synthesis and Cell-Imaging Applications of Glutathione-Capped CdTe Quantum Dots. Adv Mater 19(3):376–380. doi:10.1002/adma.200600342
Lin YH, Tseng WL (2009) Highly sensitive and selective detection of silver ions and silver nanoparticles in aqueous solution using an oligonucleotide-based fluorogenic probe. Chem Commun 45(43):6619–6621. doi:10.1039/b915990h
Zheng A, Chen J, Li H, He C, Wu G, Zhang Y, Wei H, Wu G (2009) Highly sensitive fluorescence determination of silver ions based on functionalized cadmium telluride nanorods. Microchim Acta 165(1–2):187–194. doi:10.1007/s00604-008-0119-x
Wang J, Liang J, Sheng Z, Han H (2009) A novel strategy for selective detection of Ag+ based on the red-shift of emission wavelength of quantum dots. Microchim Acta 167(3–4):281–287. doi:10.1007/s00604-009-0244-1
Liu ZQ, Liu SP, Yin PF, He YQ (2012) Fluorescence enhancement of CdTe/CdS quantum dots by coupling of glyphosate and its application for sensitive detection of copper ion. Anal Chim Acta 745:78–84. doi:10.1016/j.aca.2012.07.033
Acknowledgments
We acknowledge the Natural Science Foundation of China (No. 21277149), Zhejiang Provincial Natural Science Foundation of China (No. LR13B050001) and the Starting Research Fund of “Team Talent” from NIMTE (No. Y20402RA03) for supporting this work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 154 kb)
Rights and permissions
About this article
Cite this article
Jiao, H., Zhang, L., Liang, Z. et al. Size-controlled sensitivity and selectivity for the fluorometric detection of Ag+ by homocysteine capped CdTe quantum dots. Microchim Acta 181, 1393–1399 (2014). https://doi.org/10.1007/s00604-014-1276-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1276-8