Abstract
Purpose
This study aimed to evaluate the impact of a topical lotion (CG428) on hair thickness and density in breast cancer survivors with permanent chemotherapy-induced alopecia (PCIA).
Methods
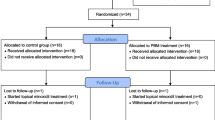
The study was a double-blind, randomized controlled trial which conducted from February 2016 to December 2016 at the Samsung Comprehensive Cancer Center in Seoul, South Korea. Breast cancer patients with PCIA were randomized on average of 3.5 years after chemotherapy. Topical lotion (Batch DT023) is a botanical drug under development containing a novel patented blend of 4 botanical ingredients: citrus, cocoa, guarana, and onion. Participants were asked to self-apply the study product or placebo twice per day for 6 months. Changes in hair density and thickness were assessed using a noninvasive bioengineering device, and patient-reported outcomes were evaluated at 3 and 6 months after randomization.
Results
A total of 35 patients were randomized to intervention (N = 18) or placebo (N = 17). Patients in the intervention group were older than those in the placebo group (52.1 vs. 41.6 years; P < 0.001). The mean hair density (SD) at baseline was 97.6 (6.4) and 126.8 (30.3) hairs/cm2 in the intervention and placebo group, respectively (P = 0.005). The corresponding values for hair thickness were 49.9 (12.7) and 48.1 (8.4) μm, respectively. After 6 months, hair density had increased by 34.7 and 24.9% compared with baseline in the intervention and control groups, respectively (P = 0.37). Corresponding values for hair thickness were 19.8 and 35.6%, respectively (P = 0.23). Similar findings were observed after age adjustment.
Discussion
In this pilot randomized clinical trial, we observed safety, tolerability, and a trend toward the efficacy of CG428 vs. placebo, especially regarding hair density and self-reported improvement.


Similar content being viewed by others
References
Lemieux J, Maunsell E, Provencher L (2008) Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology 17:317–328
Freites-Martinez A, Shapiro J, van den Hurk C, Goldfarb S, Jimenez J, Rossi AM, Paus R, Lacouture ME (2018) CME part 2: hair disorders in cancer survivors persistent chemotherapy-induced alopecia, persistent radiotherapy–induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery. J Am Acad Dermatol 80:1199–1213
Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, Lee SK, Im YH, Park YH, Yang JH, Cho J (2014) Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology 23:1103–1110
Nakashima-Kamimura N, Nishimaki K, Mori T, Asoh S, Ohta S (2008) Prevention of chemotherapy-induced alopecia by the anti-death FNK protein. Life Sci 82:218–225
Katsimbri P, Bamias A, Pavlidis N (2000) Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36:766–771
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S, Calori A, Grasso B, Volpone C, Bertola G, Parola G, Tealdi G, Giuliano PL, Aglietta M, Ballari AM (2016) Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol 2:445–452
Paus R, Haslam IS, Sharov AA, Botchkarev VA (2013) Pathobiology of chemotherapy-induced hair loss. Lancet Oncol 14:e50–e59
Sousa LP, Alessandri AL, Pinho V, Teixeira MM (2013) Pharmacological strategies to resolve acute inflammation. Curr Opin Pharmacol 13:625–631
Niu J, Azfer A, Kolattukudy PE (2006) Monocyte-specific Bcl-2 expression attenuates inflammation and heart failure in monocyte chemoattractant protein-1 (MCP-1)-induced cardiomyopathy. Cardiovasc Res 71:139–148
Badrichani AZ, Stroka DM, Bilbao G, Curiel DT, Bach FH, Ferran C (1999) Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-kappaB. J Clin Invest 103:543–553
Takeda A, Sato A, Zhang L, Harti S, Liu GCAJ (2013) CG210 enables finasteride 1mg users to further improve hair pattern: a randomized, double-blind, placebo-controlled pilot study. Hair Ther Transplant 3:1–5
Shiiba T, Kondo R, Harti S, Mello A, Cauwenbergh G, Liu J (2015) Poster session (Sunday, 27 September). Eur J Cancer 51:S225
Kang D, Kim IR, Im YH, Park YH, Ahn JS, Lee JE, Nam SJ, Park H, Kim E, Lee HK, Lee DY, Cho J (2015) Quantitative changes in skin composition parameters due to chemotherapy in breast cancer patients: a cohort study. Breast Cancer Res Treat 152:675–682
Kang D, Kim IR, Choi EK, Im YH, Park YH, Ahn JS, Lee JE, Nam SJ, Lee HK, Park JH, Lee DY, Lacouture ME, Guallar E, Cho J Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study. LID - theoncologist.2018–0184 [pii] LID - https://doi.org/10.1634/theoncologist.2018-0184
Lee SH, Kang JS, Jeon IK, Lee HS, Cho SB (2013) Two-point scoring method for the evaluation of pattern hair loss by phototrichogram using a headband and a tapeline. Skin Res Technol 19:183–188
Institute NC (2009) Common terminology criteria for adverse events, version 4.0. In: editor (ed)^(eds) book common terminology criteria for adverse events, version 4.0. National Cancer Institute, National Institutes of Health, Department of Health and Human Services; May 29, 2009 National Institutes of Health publication 09-7473, City
Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Komarov PG, Maurer M, Gilchrest BA, Gudkov AV (2000) p53 is essential for chemotherapy-induced hair loss. Cancer Res 60:5002–5006
Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, Trancik RJ, Bandstra BA, Compton LD (1996) A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol 35:74–78
Kluger N, Jacot W, Frouin E, Rigau V, Poujol S, Dereure O, Guillot B, Romieu G, Bessis D (2012) Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol 23:2879–2884
Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, Connelly EA, Chen Q, Zou J, Goldenberg C, Voellmy R (2008) Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones 13:31–38
Cece R, Cazzaniga S, Morelli D, Sfondrini L, Bignotto M, Menard S, Colnaghi MI, Balsari A (1996) Apoptosis of hair follicle cells during doxorubicin-induced alopecia in rats. Lab Investig 75:601–609
Nangia J (2018) Quality of life matters: it is time to integrate scalp cooling in routine clinical practice. J Oncol Pract 14:157–158
Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, Holmes F, Abraham J, Lacouture M, Courtright J, Paxman R, Rude M, Hilsenbeck S, Osborne CK, Rimawi M (2017) Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. Jama 317:596–605
Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, Holmes F, Abraham J, Lacouture M, Courtright J, Paxman R, Rude M, Hilsenbeck S, Osborne CK, Rimawi M (2017) Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the scalp randomized clinical trial. JAMA 317:596–605
Jimenez JJ, Yunis AA (1992) Protection from chemotherapy-induced alopecia by 1,25-dihydroxyvitamin D3. Cancer Res 52:5123–5125
Hidalgo M, Rinaldi D Fau -Medina G, Medina G Fau - Griffin T, Griffin T Fau - Turner J, Turner J Fau - Von Hoff DD, Von Hoff DD. A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D3) to prevent chemotherapy-induced alopecia
Bleiker TO, Nicolaou N Fau - Traulsen J, Traulsen J Fau - Hutchinson PE, Hutchinson PE ‘Atrophic telogen effluvium’ from cytotoxic drugs and a randomized controlled trial to investigate the possible protective effect of pretreatment with a topical vitamin D analogue in humans
Taylor M, Ashcroft AT, Messenger AG (1993) Cyclosporin A prolongs human hair growth in vitro. J Invest Dermatol 100:237–239
Hawkshaw NJ, Haslam IS, Ansell DM, Shamalak A, Paus R (2015) Re-evaluating cyclosporine a as a hair growth-promoting agent in human scalp hair follicles. J Invest Dermatol 135:2129–2132
Dunnill CJ, Al-Tameemi W, Collett A, Haslam IS, Georgopoulos NT (2018) A clinical and biological guide for understanding chemotherapy-induced alopecia and its prevention. Oncologist 23:84–96
Glaser DA, Hossain P, Perkins W, Griffiths T, Ahluwalia G, Weng E, Beddingfield FC (2015) Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomized controlled trial. Br J Dermatol 172:1384–1394
Cucé LC, Rodrigues C, Patriota RCR (2011) Cellium® GC: evaluation of a new natural active ingredient in 210 mg/ml topical solution, through scalp biopsy, pp 123-128
Kim SN, Lee SY, Choi MH, Joo KM, Kim SH, Koh JS, Park WS (2013) Characteristic features of ageing in Korean women’s hair and scalp. Br J Dermatol 168:1215–1223
Funding
This work was supported by Legacy Healthcare and Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03031654).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
The sponsor was not involved in the conduct of the study, collection of the data, or statistical analysis.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Kang, D., Kim, IR., Park, Y.H. et al. Impact of a topical lotion, CG428, on permanent chemotherapy-induced alopecia in breast cancer survivors: a pilot randomized double-blind controlled clinical trial (VOLUME RCT). Support Care Cancer 28, 1829–1837 (2020). https://doi.org/10.1007/s00520-019-04982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04982-z