Abstract
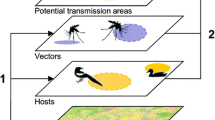
Japanese encephalitis (JE) is a vector-borne disease transmitted by mosquitoes and maintained in birds and pigs. To examine the possible epidemiology of JE in the United States, we use an individual-level network model that explicitly considers the feral pig population and implicitly considers mosquitoes and birds in specific areas of Florida, North Carolina, and South Carolina. To model the virus transmission among feral pigs within a small geographic area (<60 sq mi areas), two network topologies are considered: Fully connected and Erdos–Renyi networks. Long-distance connections (interstate) are created with limited probability and based on fall and spring bird migration patterns. Patterns of simulated outbreaks support the use of the Erdos–Renyi network because maximum incidence occurs during the fall migration period which is similar to the peak incidence of the closely related West Nile virus, another virus in the Japanese encephalitis group (Flaviviridae) that is transmitted by both birds and mosquitoes. Simulation analysis suggested two important mitigation strategies: for low mosquito vectorial capacity, insecticidal spraying of infected areas reduces transmission and limits the outbreak to a single geographic area. Alternatively, in high mosquito vectorial capacity areas, birds rather than mosquitoes need to be removed/controlled.







Similar content being viewed by others
References
Agarwal M, Verma V (2012) The impact of media on the spreading and control of Japanese encephalitis. Int J Math Sci Comput 2(2):23–31
Biology (2015) What is the difference between a mechanistic and a statistical model? http://biology.stackexchange.com/questions/34231/what-is-the-difference-between-a-mechanistic-and-a-statistical-predictive-model
Britton T, Giardina F (2016) Introduction to statistical inference for infectious diseases. J Soc Fr Stat 157(1):53–70
Brodland GW (2015) How computational models can help unlock biological systems. Seminars in cell and developmental biology, vol 73., p 67
Buescher EL, Scherer WF (1959) Ecologic studies of Japanese encephalitis virus in Japan. IX. Epidemiologic correlations and conclusions. Am J Trop Med Hyg 8:719–722
Burke DS, Tingpalapong M, Ward GS, Andre R, Leake CJ (1985) Intense transmission of Japanese encephalitis virus to pigs in a region free of epidemic encephalitis. Southeast Asian. J Trop Med Public Health 16:199–206
Ciota AT, Drummond CL, Ruby MA, Drobnack J, Ebel GD, Kramer LD (2012) Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J Med Entomol 49(1):35–42
Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P (2011) Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet 377(9765):599–609
Desouza K, Yuan L (2013) Towards evidence-driven policy design: complex adaptive systems and computational modeling. Annu Rev Policy Des 1:1–19
eBird (2015) Cornell lab of ornithology. http://ebird.org/ebird/places?yr=allandm. Accessed 23 Mar 2016
Erdos P, Rényi A (1960) On the evolution of random graphs. Publ Math Inst Hungar Acad Sci 5:17–61
Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K (2009) Past, present, and future of Japanese encephalitis. Emerg Infect Dis 15(1):1–7
Evans MR, Bithell M, Cornell SJ, Dall SRX, Díaz S, Emmott S, Ernande B, Grimm V, Hodgson DJ, Lewis SL, Mace GM, Morecroft M, Moustakas A, Murphy E, Newbold T, Norris KJ, Petchey O, Smith M, Travis JMJ, Benton TG (2013) Predictive systems ecology. Proc R Soc B 280:20131452
Evans MR, Benton TG, Grimm V, Lessells CM, O’Malley MA, Moustakas A, Weisberg M (2014) Data availability and model complexity, generality, and utility: a reply to Lonergan. Trends Ecol Evol 29:302–303
Garrett-Jones C (1964) The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ 30(2):241
Garrett-Jones C, Shidrawi GR (1969) Malaria vectorial capacity of a population of Anopheles gambiae: an exercise in epidemiological entomology. Bull World Health Organ 40(4):531
Gunasekaran K, Sahu SS, Jambulingam P (2014) Estimation of vectorial capacity of Anopheles minimus Theobald and An. fluviatilis James (Diptera: Culicidae) in a malaria endemic area of Odisha State, India. Indian J Med Res 140(5):653
Handel A, Longini IM, Antia R (2007) What is the best control strategy for multiple infectious disease outbreaks? Proc R Soc Lond B 274(1611):833–837
Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ (1995) An outbreak of Japanese encephalitis in the Torres Strait, Australia. Med J Aust 165(5):256–260
Honeyman MS (2005) Extensive bedded indoor and outdoor pig production systems in USA: current trends and effects on animal care and product quality. Livest Prod Sci 94(1):15–24
Huang YJ, Harbin JN, Hettenbach SM, Maki E, Cohnstaedt LW, Barrett AD, Higgs S, Vanlandingham DL (2015) Susceptibility of a North American Culex quinquefasciatus to Japanese encephalitis virus. Vector Borne Zoonotic Dis 15(11):709–711
Jourdain E, Gauthier-Clerc M, Bicout DJ, Sabatier P (2007) Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg Infect Dis 13(3):365
Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9(3):311–322
Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286(5448):2333–2337
Mackenzie JS, Gubler DJ, Petersen LR (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10:S98–S109
Manore C, Hyman M (2016) Mathematical models for fighting Zika virus. Siam News, Philadelphia
Moustakas A, Evans MR (2015) Coupling models of cattle and farms with models of badgers for predicting the dynamics of bovine tuberculosis (TB). Stoch Environ Res Risk Assess 29(3):623–635
Mukhopadhyay BB, Tapaswi PK (1994) An SIRS epidemic model of Japanese encephalitis. Int Journal of Math Math Sci 17(2):347–355
Naresh R, Pandey S (2009) Modeling and analysis of the spread of Japanese encephalitis with environmental effects. Appl Appl Math 4(1):155–175
Newton I (2010) The migration ecology of birds. Academic Press, Cambridge
Owen J, Moore F, Panella N, Edwards E, Bru R, Hughes M, Komar N (2006) Migrating birds as dispersal vehicles for West Nile virus. Eco Health 3(2):79–85
Peterson AT, Vieglais DA, Andreasen JK (2003) Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector-Borne Zoonotic Dis 3(1):27–37
Rappole J, Derrickson SR, Hubálek Z (2000) Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis 6(4):319–328
Rappole JH, Compton BW, Leimgruber P, Robertson J, King DI, Renner SC (2006a) Modeling movement of West Nile virus in the Western hemisphere. Vector Borne Zoonotic Dis 6(2):128–139
Rappole JH, Derrickson SR, Hubálek Z (2006b) Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis 6(4):319
Reed KD, Meece JK, Henkel JS, Shukla SK (2003) Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin Med Res 1(1):5–12
Reiner RC, Perkins TA, Barker CM, Niu T, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam JR, Bisanzio D, Buckee C (2013) A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J R Soc Interface 10(81):20120921
Ricklin ME, García-Nicolás O, Brechbühl D, Python S, Zumkehr B, Nougairede A, Charrel RN, Posthaus H, Oevermann A, Summerfield A (2016) Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat commun 23:7
Sahneh FD, Scoglio C, Van Mieghem P (2013) Generalized epidemic mean-field model for spreading processes over multilayer complex networks. IEEE/ACM Trans Netw 21(5):1609–1620
Sahneh FD, Vajdi A, Shakeri H, Fan F., Scogilo C (2016) GEMFsim: a stochastic simulator for the generalized epidemic modeling framework. https://arxiv.org/1528946. Accessed 7 Apr 2016
Schuyler PT, Garcelon DK, Escover S (2002) Eradication of feral pigs (Sus scrofa) on Santa Catalina island, California, USA. Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group, Gland, p 274
SCWDS (2015) Feral swine distribution map. http://swine.vet.uga.edu/nfsms/information/map2015.htm. Accessed 26 Mar 2016
SE Corporation wildlife disease study (1988) Feral/wild swine populations. http://vet.uga.edu/population_health_files/scwds-150swine88-2012.jpg. Accessed 26 Mar 2016
SEWISC (2007) Southeastern Wisconsin invasive species consortium Inc. http://sewisc.org/invasives/invasive-animals/59-feral-pig. Accessed 26 Mar 2016
Solomon T (2006) Control of Japanese encephalitis—within our grasp? N Engl J Med 355:869–871. doi:10.1056/NEJMp058263
Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD (2003) Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol 77(5):3091–3098
Tapaswi PK, Ghosh AK, Mukhopadhyay BB (1995) Transmission of Japanese encephalitis in a 3-population model. Ecol Model 83(3):295–309
Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MP (2009) Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J R Soc Interface 6(31):187–202
United Nations (2005) The United Nations urbanization prospects: the 2005 revision. POP/DB/WUP/Rev.2005/1/F1. United Nations, New York
Unkel S, Farrington C, Garthwaite PH, Robertson C, Andrews N (2012) Statistical methods for the prospective detection of infectious disease outbreaks: a review. J R Stat Soc 175(1):49–82
USGS (2015) West Nile virus human provisional 2015 data. http://diseasemaps.usgs.gov/mapviewer/. Accessed 26 Mar 2016
Vythilingam I, Chiang GL, Lee HL, Singh K (1992) Special report on bionomics of important mosquito vectors in Malaysia. Southeast Asian J Trop Med Public Health 23(4):581–602
Weaver SC, Barrett AD (2004) Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol 2(10):789–801
Williams DT, Daniels PW, Lunt RA, Wang LF, Newberry KM, Mackenzie JS (2001) Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. Am J Trop Med Hyg 65(4):379–387
World Health Organization (2013) World health report: Research for universal health coverage. http://www.who.int/whr/en. Accessed 26 Mar 2016
Yu HL, Yang SJ, Yen HJ, Christakos G (2011) A spatio-temporal climate-based model of early dengue fever warning in southern Taiwan. Stoch Env Res Risk Assess 25(4):485–494
Funding
This material is based upon work supported by the United States Department of Agriculture Research Project #427647, and by the National Science Foundation under Grant No. CIF-1423411. The views and conclusions contained in this publication are those of the authors and should not be interpreted as necessarily representing the official policies, either explicit or implicit, of the United States Department of Agriculture. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riad, M.H., Scoglio, C., McVey, D.S. et al. An individual-level network model for a hypothetical outbreak of Japanese encephalitis in the USA. Stoch Environ Res Risk Assess 31, 353–367 (2017). https://doi.org/10.1007/s00477-016-1353-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-016-1353-0