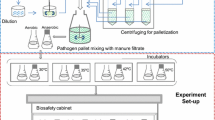
Abstract
To assess Escherichia coli (E. coli) persistence in dairy manure, bench scale experiments were conducted under aerobic and anaerobic environments. The changes in E. coli levels in dairy manure were assessed at moderate (25 °C), mesophilic (37 °C), and thermophilic (52.5 °C) temperatures. The inactivation of E. coli at moderate, mesophilic, and thermophilic temperatures were described by linear regression equations. Subsequently, double-exponential kinetic models were developed to describe the E. coli decay curves under aerobic and anaerobic environments. The kinetics models were used to estimate E. coli log reductions at various temperatures. Results showed that the double-exponential kinetic models performed well while calculating E. coli reductions in dairy manure over the incubation period. In addition, we evaluated digestate to compare the changes in total solids and volatile solids, total organic carbon, total nitrogen, pH, and oxygen reduction potential levels in aerobic and anaerobic conditions under various temperatures. We anticipate that the results presented here will be useful for enhancing the understanding of pathogen reduction in anaerobic and aerobic processes during dairy manure treatment.





Similar content being viewed by others
Change history
09 April 2018
Escherichia coli persistence kinetics in dairy manure at moderate, mesophilic, and thermophilic temperatures.
References
United States Environmental Protection Agency (USEPA) (2014). Animal Waste Management in the Pacific Southwest. Washington DC, USA. http://www.epa.gov/region9/animalwaste/index.html. Accesses on 23 June 2014
Armstrong SD, Smith DR, Joern BC, Owens PR, Leytem AB, Huang C, Adeola L (2009) Transport and fate of phosphorus during and after manure spill simulations. J Environ Qual 39:345–352
Toth JD, Aceto HW, Rankin SC, Dou Z (2013) Short communication: survey of animal borne pathogens in the farm environment of 13 dairy operations. J Dairy Sci 96(9):5756–5761
United States Environmental Protection Agency (USEPA) (2010) WATERS (Watershed Assessment, Tracking & Environmental Results). Washington, D.C
Arnone RD, Walling JP (2007) Waterborne pathogens in urban watersheds. J Water Health 5(1):149–162
Jenkins RW, Stageman NE, Fortune CM, Chuck CJ (2014) Effect of the type of bean, processing, and geographical location on the biodiesel produced from waste coffee grounds. Energy Fuels 28(2):1166–1174
Thrane C (2012) Assessment of human-health impact of Salmonella in animal feed. National Food Institute, Technical University of Denmark. http://www.food.dtu.dk. Accessed on 23 June 2014
Beaudet R, Gagnon C, Bisaillon JG, Ishaque M (1990) Microbial aspects of aerobic thermophilic treatment of swine waste. Appl Environ Microbiol 56(4):971–976
Kearney TE, Larkin MJ, Frost JP, Levett PN (1993) Survival of pathogenic bacteria during mesophilic anaerobic digestion of animal waste. J Appl Bacteriol 75:215–219
Gadre RV, Ranade DR, Godbole SH (1986) A note on survival of salmonellas during anaerobic digestion of cattle dung. J Appl Bacteriol 60:93–96
Over K, Crandall PG, O’Bryan CA, Ricke SC (2011) Current perspectives on Mycobacterium avium ssp. paratuberculosis, Johne’s disease, and Crohn’s disease: a review. Crit Rev Microbiol 37:141–156
San Joaquin Valley Dairy Manure Technology Feasibility Assessment Panel (2005) An assessment of technologies of management and treatment of dairy manure in California’s San Joaquin Valley. California, USA. http://www.arb.ca.gov/ag/caf/dairypnl/dmtfaprprt.pdf. Accessed 23 June 2014
Resende JA, Silva VL, Oliveira RLR, Fortunato SO, Carneiro JC, Otenio MH, Diniz CG (2014) Prevalence and persistence of potentially pathogenic and antibiotic resistant bacteria during anaerobic digestion treatment of cattle manure. Bioresour Technol 153:284–291
Rubia MA, Riau V, Raposo F, Borja R (2013) Thermophilic anaerobic digestion of sewage sludge: focus on the influence of the start-up. A review. Crit Rev Biotechnol 33(4):448–460
Pandey PK, Soupir ML (2011) Escherichia coli inactivation kinetics in anaerobic digestion of dairy manure under moderate, mesophilic, and thermophilic temperatures. AMB Express. doi:10.1186/2191-0855-1-18
Viancelli A, Kunz A, Steinmetz RLR, Kich JD, Souza CK, Canal CW, Coldebella A, Esteves PA, Barardi CR (2013) Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 90:1539–1544
Martens W, Böhm R (2009) Overview of the ability of different treatment methods for liquid and solid manure to inactivate pathogens. Bioresour Technol 100:5374–5378
Huang H, Spencer JL, Soutyrine A, Guan J, Rendulich J, Balachandran A (2007) Evidence for degradation of abnormal prion protein in tissues from sheep with scrapie during composting. Can J Vet Res 71:34–40
Chaney CG, St Martin, Bekele I, Eudoxie GD, Bristol D, Brathwaite RAI, Campo K (2014) Modelling response patterns of physico-chemical indicators during high-rate composting of green waste for suppression of Pythium ultimum. Environ Techno. doi:10.1080/09593330.2013.839719 39
Xu W, Reuter T, Inglis GD, Larney FJ, Alexander TW, Guan J, Stanford K, Xu Y, McAllister TA (2009) A biosecure composting system for disposal of cattle carcasses and manure following infectious disease outbreak. J Environ Qual 38:437–450
Flory GA, Peer RW (2010) Verification of poultry carcass composting research through application during actual avian influenza outbreaks. ILAR J 51:149–157
Reuter T, Alexander TW, McAllister TA (2011) Viability of Bacillus licheniformis and Bacillus thuringiensis spores as a model for predicting the fate of Bacillus anthracis spores during composting of livestock mortalities. Appl Environ Microbiol 77:1588–1592
Tkachuk VL, Krause DO, Knox NC, Hamm AC, Zvomuya F, Ominski KH, McAllister TA (2014) Targeted 16S rRNA high throughput sequencing to characterize microbial communities during composting of livestock mortalities. J Appl Microbiol. doi:10.1111/jam.12449
Lung AJ, Lin CM, Kim JM, Marshall MR, Nordstedt R, Thompson NP, Wei CI (2001) Destruction of Escherichia coli O157:H7 and Salmonella Enteritidis in cow manure composting. J Food Prot 64:1309–1314
Samadpour M, Barbour MW, Nguyen T, Cao TM, Buck F, Depavia GA, Mazengia E, Yang P, Alfi D, Lopes M, Stopforth JD (2006) Incidence of enterohemorrhagic Escherichia coli, Escherichia coli O157, Salmonella, and Listeria monocytogenes in retail fresh ground beef, sprouts, and mushrooms. J Food Prot 69:441–443
Weil JD, Cutter CN, Beelman RB, Laborde LF (2013) Inactivation of human pathogens during phase II composting of manure-based mushroom growth substrate. J Food Prot 76:1393–1400
Kabrick RM, Jewell WJ (1982) Fate of pathogens in thermophilic aerobic sludge digestion. Water Res 16:1051–1060
McGarvey JA, Miller WG, Zhang R, Ma Y, Mitloehner F (2007) Bacterial population dynamics in dairy waste during aerobic and anaerobic treatment and subsequent storage. Appl Environ Microbiol 73(1):193–202
American Public Health Association (APHA) (1999) Standard methods for the examination of water and wastewater. A.W.W.A., Water Environment Federation
Carter MR, Gregorich EG (1993) Soil sampling and methods of analysis. Lewis, Boca Raton, Florida, USA
Nicholson FA, Groves SJ, Chambers BJ (2005) Pathogen survival during livestock manure storage and following land application. Bioresour Technol 96:135–143
Prochazka J, Dolejs P, Maca J, Dohanyos M (2012) Stability and inhibition of anaerobic processes caused by insufficiency of excess of ammonia nitrogen. Appl Microbiol Biotechnol 93:439–447
Miqueleto AP, Dolosic CC, Pozzi E, Zaiat M (2010) Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour Technol 101:1324–1330
Mengistu Y, Edwards C, Saunders JR (1994) Continuous culture studies on the synthesis of capsular polysaccharide by Klebsiella pneumoniae K1. J Appl Bacteriol 76:424–430
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Mol Biol Rev 55(1):35–58
Popat SC, Yates MV, Deshusses MA (2010) Kinetics of inactivation of indicator pathogens during thermophilic anaerobic digestion. Water Res 44(20):5965–5972
Aitken MD, Sobsey MD, Blauth KE, Shehee M, Crunk PL, Walters GW (2005) Inactivation of Ascaris suum and Poliovirus in Biosolids under thermophilic anaerobic digestion conditions. Environ Sci Technol 39(15):5804–5809
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305:1622–1625
Feng J, Kessler DA, Ben-Jacob E, Levine H (2013) Growth feedback as a basis for persister bistability. PNAS 111(1):544–549
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Maisonneuve E, Shakespeare LJ, Jorgensen MG, Gerdes K (2011) Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci USA 108(32):13206–13211
Praveen C, Jesudhasan PR, Reimers RS, Pillai SD (2013) Electron beam inactivation of selected microbial pathogens and indicator organisms in aerobically and anaerobically digested sewage sludge. Bioresour Technol 144:652–657
Winkler MKH, Brennenbroek MH, Horstink FH, Van Loosdrecht MCM, Van de Pol GJ (2013) The biodrying concept: an innovative technology creating energy from sewage sludge. Bioresour Technol 147:124–129
Lloret E, Pastor L, Pradas P, Pascual JA (2013) Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: stabilization process and pathogen reduction. Chem Eng J 232:42–50
Franke-Whittle IH, Insam H (2013) Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: a review. Crit Rev Microbiol 39(2):139–151
Bagge E, Persson M, Johansson KE (2010) Diversity of spore-forming bacteria in cattle manure, slaughterhouse waste and samples from biogas plants. J Appl Microbiol 109:1549–1565
Gobec I, Ocepek M, Pogacnik M, Dobeic M (2009) Inactivation of Mycobacterium avium paratuberculosis in sheep manure. Slov Vet Res 46:105–113
Jenkins MB, Truman CC, Siragusa G, Line E, Bailey JS, Frye J, Endale DM, Franklin DH, Schomberg HH, Fisher DS, Sharpe RR (2008) Rainfall and tillage effects on transport of fecal bacteria and sex hormones 17β-estradiol and testosterone from broiler litter applications to a Georgia Piedmont Ultisol. Sci Total Environ 403:154–163
Soupir ML, Mostaghimi S, Yagow ER, Hagedorn C, Vaughan DH (2006) Transport of fecal bacteria from poultry litter and cattle manures applied to pastureland. Water Air Soil Pollut 169:125–136
Mallin MA, Cahoon LB (2003) Industrialized animal production-a major source of nutrient and microbial pollution to aquatic ecosystems. Popul Environ 24(5):369–385
Han I, Congeevaram S, Ki DW, Oh BT, Park J (2011) Bacterial community analysis of swine manure treated with auto thermal thermophilic aerobic digestion. Appl Microbiol Biotechnol 89:835–842
Jiang X, Morgan J, Doyle MP (2003) Fate of Escherichia coli O157:H7 during composting of bovine manure in a laboratory-scale bioreactor. J Food Prot 66:25–30
NABC (2004) Carcass disposal: a comprehensive review. Report written for the USDA Animal and Plant Health Inspection Service. National Agricultural Biosecurity Centre, Kansas State University, USA
Slana I, Pribylova R, Kralova A, Pavlik I (2011) Persistence of Mycobacterium avium subsp. paratuberculosis at a farm-scale biogas plant supplied with manure from paratuberculosis-affected dairy cattle. Appl Environ Microbiol 77:3115–3119
Grewal SK, Rajeev S, Sreevatsan S, Michel FC Jr (2006) Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl Environ Microbiol 72:565–574
Acknowledgments
Authors thank to Division of Agriculture and Natural Resources (ANR), University of California, Davis, and Iowa State University, Ames for supporting the work. The authors would like to thank Kendal Agee, Andrew Paxson, Charles Velasquez, and Ray Sims, students and trainees of Iowa State University, Ames, Iowa, for assistance with sample collection and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, P.K., Biswas, S., Vaddella, V.K. et al. Escherichia coli persistence kinetics in dairy manure at moderate, mesophilic, and thermophilic temperatures under aerobic and anaerobic environments. Bioprocess Biosyst Eng 38, 457–467 (2015). https://doi.org/10.1007/s00449-014-1285-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1285-3