Abstract
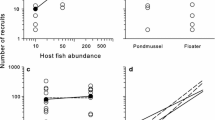
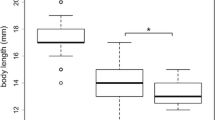
Natural populations often face multiple mortality sources. Adaptive responses to one mortality source might also be beneficial with respect to other sources of mortality, resulting in “reinforcing adaptations”; or they might be detrimental with respect to other sources of mortality, resulting in “conflicting adaptations”. We explored these possibilities by testing experimentally if the responses of guppies (Poecilia reticulata) to the monogenean ectoparasitic worm Gyrodactylus differed between populations adapted to different predation regimes. In experimental stream channels designed to replicate the natural environment, we exposed eight guppy populations (high-predation and low-predation populations from each of four separate rivers) either to their local Gyrodactylus parasites (infection treatment) or to the absence of those parasites (control). We found that infection dynamics varied dramatically among populations in a repeatable fashion, but that this variation was not related to the predation regime of origin. Consistent with previous work, high-predation guppy females gained more mass, had lower reproductive investment, and had more but smaller embryos than did low-predation females. Relative to control (no parasite) channels, guppies from treatment (infected) channels gained less mass but produced similar numbers and sizes of embryos—and thus had a higher reproductive effort. However, no interaction was evident between infection treatment and predation regime. We conclude that parasitism by Gyrodactylus and predation are both likely selective forces for guppies, but that adaptation to predation does not have an obvious deterministic effect on host–parasite dynamics or on life-history traits of female guppies.



Similar content being viewed by others
References
Arendt JD, Reznick DN (2005) Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc R Soc Lond B 272:333–337
Bashey F (2006) Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Evolution 60:348–361
Bassar RD, Marshall MC, Lopez-Sepulcre A, Zandona E, Travis J, Pringle CM, Flecker AS, Thomas SA, Fraser DF, Reznick DN (2010) Local adaptation in Trinidadian guppies alters ecosystem processes. Proc Natl Acad Sci USA 107(8):3616–3621
Billoir E, Péry ARR, Charles S (2007) Integrating the lethal and sublethal effects of toxic compounds into the population dynamics of Daphnia magna: a combination of the DEBtox and matrix population models. Ecol Model 203:204–214
Bryant MJ, Reznick DN (2004) Comparative studies of senescence in natural populations of guppies. Am Nat 163:55–68
Cable J, van Oosterhout C (2007a) The impact of parasites on the life history evolution of guppies (Poecilia reticulata): the effects of host size on parasite virulence. Int J Parasitol 37:1449–1458
Cable J, van Oosterhout C (2007b) The role of innate and acquired resistance in two natural populations of guppies (Poecilia reticulata) infected with the ectoparasite Gyrodactylus turnbulli. Biol J Linn Soc 90:647–655
Dybdahl MF, Storfer A (2003) Parasite local adaptation: Red Queen versus Suicide King. Trends Ecol Evol 18(3):523–530
Dzikowski R, Hulata G, Harpaz S, Karplus I (2004) Inducible reproductive plasticity of the guppy Poecilia reticulata in response to predation cues. J Exp Zool 301A:776–782
Ebert D, Hamilton WD (1996) Sex against virulence: the coevolution of parasitic diseases. Trends Ecol Evol 11:79–82
Endler JA (1978) A predator’s view of animal color patterns. Evol Biol 11:319–364
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34(1):76–91
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol 10(1):22–29
Fajen A, Breden F (1992) Mitochondrial DNA sequence variation among natural populations of the Trinidad guppy, Poecilia reticulata. Evolution 46:1457–1465
Faria PJ, van Oosterhout C, Cable J (2010) Optimal release strategies for captive-bred animals in reintroduction programs: experimental infections using the guppy as a model organism. Conserv Biol 143:35–45
Forbes M (1993) Parasitism and host reproductive effort. Oikos 67(3):444–450
Fraser B, Neff BD (2010) Parasite mediated homogenizing selection at the MHC in guppies. Genetica 138:273–278
Fraser B, Ramnarine IW, Neff BD (2010) Temporal variation at the MHC class IIB in wild populations of the guppy (Poecilia Reticulata). Evolution 64(7):2086–2096
Gandon S, Nuismer SL (2009) Interactions between genetic drift, gene flow, and selection mosaics drive parasite local adaptation. Am Nat 173:212–224
Gliwicz J (2007) Increased reproductive effort as a life history response of Microtus to predation. Ecoscience 14:314–317
Gordon SP, Reznick ND, Kinnison MT, Bryant MJ, Weese DJ, Rasanen K, Millar NP, Hendry AP (2009) Adaptive changes in life history and survival following a new guppy introduction. Am Nat 174(1):34–45
Gosline AK, Rodd FH (2008) Predator-induced plasticity in guppy (Poecilia reticulata) life history traits. Aquat Ecol 42:693–699
Harris PD, Lyles AM (1992) Infections of Gyrodactylus bullatarudis and Gyrodactylus turnbulli on guppies (Poecilia reticulata) in Trinidad. J Parasitol 78:912–914
Harris PD, Shinn AP, Cable J, Bakke TA (2004) Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Syst Parasitol 59(1):1–27
Hatcher MJ, Dick JTA, Dunn A (2006) How parasites affect interactions between competitors and predators. Ecol Lett 9:1253–1271
Haynes JL (1995) Standardized classification of poeciliid development for life-history studies. Copeia 1995:147–154
Holland JN, DeAngelis DL (2009) Consumer-resource theory predicts dynamic transitions between outcomes of interspecific interactions. Ecol Lett 12:1357–1366
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, Princeton
Karim N, Gordon SP, Schwartz AK, Hendry AP (2007) This is not déjà vu all over again: male guppy colour in a new experimental introduction. J Eur Soc Evol Biol 20:1–12
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241
Kearn GC (1994) Evolutionary expansion of the monogenea. Int J Parasitol 24:1227–1271
Kolluru GR, Ruiz NC, del Cid N, Dunlop E, Grether GF (2006) The effects of carotenoid and food intake on caudal fin regeneration in male guppies. J Fish Biol 68:1002–1012
Kolluru GR, Grether GF, Dunlop E, South S (2009) Food availability and parasite infection influence mating tactics in guppies (Poecilia reticulata). Behav Ecol 20(1):131–137
Law R (1979) Optimal life histories under age-specific predation. Am Nat 114:399–417
Lively CM (1999) Migration, virulence, and the geographic mosaic of adaptation by parasites. Am Nat 153:S34–S47
Magurran AE (2005) Evolutionary ecology the Trinidadian guppy. Oxford University Press, Oxford
Magurran AE, Phillip DAT (2001) Evolutionary implications of large-scale patterns in the ecology of Trinidadian guppies, Poecilia reticulata. Biol J Linn Soc 73:1–9
Martin CH, Johnsen S (2007) A field test of the Hamilton-Zuk hypothesis in the Trinidadian guppy (Poecilia reticulata). Behav Ecol Sociobiol 61:1897–1909
Millar NP, Hendry AP (2011) Population divergence of private and non-private signals in wild guppies. Environ Biol Fish (in press)
Mouritsen KN, Poulin R (2010) Parasitism as a determinant of community structure on intertidal flats. Mar Biol 157(1):201–213
Nuismer SL, Gandon S (2008) Moving beyond common-garden and transplant designs: insight into the causes of local adaptation in species interactions. Am Nat 171:658–668
Palkovacs EP, Marshall MC, Lamphere BA, Lynch BR, Weese DJ, Fraser DF, Reznick DN, Pringle CM, Kinnison MT (2009) Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philos Trans R Soc Lond B 364:1617–1628
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton
R Core Development Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reznick DN (1982) The impact of predation on life history evolution in Trinidadian guppies: genetic basis of observed life history patterns. Evolution 36(6):1236–1250
Reznick DN (1989) Life-history evolution in guppies: 2. Repeatability of field observations and the effects of season on life histories. Evolution 43(6):1285–1297
Reznick DN, Bryga H (1987) Life-history evolution in guppies (Poecilia reticulata): 1. Phenotypic and genetic changes in an introduction experiment. Evolution 41(6):1370–1385
Reznick DN, Bryga H (1996) Life-History evolution in guppies (Poecilia reticulata: Poeciliidae). V. Genetic basis of parallelism in life histories. Am Nat 147(3):339–359
Reznick DN, Endler J (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36(1):160–177
Reznick DN, Butler MJ IV, Rodd FH, Ross P (1996) Life-history evolution in guppies (Poecilia reticulata) 6. Differential mortality as a mechanism for natural selection. Evolution 50(4): 1651-1660
Rodd FH, Reznick DN (1997) Variation in the demography of guppy populations: the importance of predation and life histories. Ecology 78(2):405–418
Roff DA (1992) The evolution of life histories. Chapman & Hall, New York
Scott ME (1982) Reproductive potential of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology 85:217–236
Scott ME (1985) Dynamics of challenge infections of Gyrodactulus bullatarudis Turbull (Monogenea) on guppies, Poecilia reticulata (Peters). J Fish Dis 8:495–503
Scott ME, Anderson RM (1984) The population dynamics of Gyrodactylus bullatarudis (Monogenea) within laboratory populations of the fish host Poecilia reticulata. Parasitology 89:159–194
Seghers BH (1974) Schooling behavior in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28:486–489
Seghers BH, Magurran AE (1995) Population differences in the schooling behavior of the Trinidadian guppy, Poecilia reticulata—adaptation or constraint. Can J Zool 73:1100–1105
Stearns SC (1977) The evolution of life history traits: a critique to the theory and a review of the data. Annu Rev Ecol Syst 8:145–171
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stibor H (1992) Predator induced life-history shifts in a freshwater cladoceran. Oceologia 92:162–165
Stoks R, McPeek MA (2003) Predators and life histories shape Lestes damselfly assemblages along a freshwater habitat gradient. Ecology 84(6):1576–1587
van Oosterhout C, Harris PD, Cable J (2003) Marked variation in parasite resistance between two wild populations of the Trinidadian guppy, Poecilia reticulata (Pisces: Poeciliidae). Biol J Linn Soc 79(4):645–651
van Oosterhout C, Domino AJ, Cummings SM, Blais J, Barson NJ, Ramnarine IW, Mohammed RS, Persad N, Cable J (2006) Balancing genetic drift, and genetic variation at the Major Histocompatibility Complex in two wild populations of guppies (Poecilia reticulata). Evolution 60(12):2562–2574
van Oosterhout C, Mohammed RS, Hansen H, Archard GA, McMullan M, Weese DJ, Cable J (2007) Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata). Int J Parasitol 37(7):805–812
van Valen L (1973) A new evolutionary law. Evol Theory 1:1–30
Weese DJ, Gordon SP, Hendry AP, Kinnison MT (2010) Spatiotemporal variation in linear natural selection on body color in wild guppies (Poecilia reticulata). Evolution 64:1802–1815
Willing EM, Bentzen P, van Oosterhout C, Hoffmann M, Cable J, Breden F, Weigel D, Dreyer C (2010) Genome-wide single nucleotide polymorphisms reveal population history and adaptive divergence in wild guppies. Mol Ecol 19:968–984
Yin M, Laforsch C, Lohr JN, Wollinska J (2011) Predator-induced defense makes Daphnia more vulnerable to parasites. Evolution 65(6):1482–1488
Acknowledgments
We would like to thank Alfredo Marquez for his help with fish collection and parasite sampling. We also thank David Reznick and the FIBR Program (From Genes to Ecosystems) for facilitating use of the experimental channels, valuable suggestions, and overall support. Felipe Dargent and Etienne Low-Decarie provided valuable comments and discussions. Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) in the form of a Special Research Opportunity Grant to G. Fussmann, A. Hendry, P. Bentzen and M. Scott, Discovery Grants to M. Scott, and PhD scholarship from the Consejo Nacional de Ciencia y Tecnología (Mexico) to F. Pérez-Jvostov. Research at the Institute of Parasitology is supported by a regroupement stratégique from Fonds Québecois de la recherche sur la nature et les technologies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joel Trexler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pérez-Jvostov, F., Hendry, A.P., Fussmann, G.F. et al. Are host–parasite interactions influenced by adaptation to predators? A test with guppies and Gyrodactylus in experimental stream channels. Oecologia 170, 77–88 (2012). https://doi.org/10.1007/s00442-012-2289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2289-9