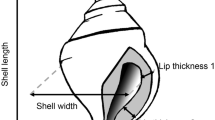
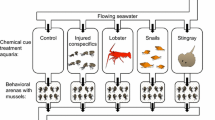
Abstract
Numerous studies demonstrate how sessile marine organisms utilize chemical, structural, and nutritional deterrents to persist in predator-rich environments. Little is known, however, about how mobile, more behaviorally complex species minimize predation by integrating avoidance and deterrence strategies. We investigated this using sabellid polychaete worms from the Caribbean and temperate western Atlantic. Sabellids extend their feather-like radioles beyond their protective tubes for feeding and respiration; the body remains inside the tube and the radioles retract when threatened. We used co-occurring consumers to determine the palatability of radioles and bodies for each of the eight species tested. In addition, we examined chemical or structural traits affecting palatability and evaluated predator escape traits, such as tube strength, speed of radiole retraction, completeness of retraction, and sensitivity to a nearby disturbance. All species had unpalatable radioles that were chemically or structurally defended, but only two species had unpalatable bodies. Thus, most species allocated defenses to tissues that were most exposed to predation. The two species with chemically defended bodies, Bispira brunnea and Bispira variegata, relied less on behavioral escapes than the other species. Their tubes were weak, they did not retract until disturbances were very close, and B. brunnea retracted slowly and incompletely even when touched. Other species generally had stronger tubes and/or retracted when disturbances were farther away. This trade-off of deterrence versus escape even occurred within a single species when populations differed in palatability. Populations of B.variegata from North Carolina and Georgia were chemically deterrent to both temperate and tropical consumers, while populations from Panama and Florida were palatable. The more palatable Panama population retracted in response to distant movement, while the unpalatable North Carolina population did not retract until nearly touched. Thus, most species utilize a combination of predator avoidance and deterrence strategies, but more deterrent populations of species utilized avoidance less.





Similar content being viewed by others
References
Amsler CD, Iken K, McClintock JB, Amsler MO, Peters KJ, Hubbard JM, Furrow FB, Baker BJ (2005) Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Penninsula. Mar Ecol Prog Ser 294:141–159
Avila C, Paul V (1997) Chemical ecology of the nudibranch Glossodoris pallida: is the location of diet-derived metabolites important for defense? Mar Ecol Prog Ser 150:171–180
Baker-Dittus AM (1978) Foraging patterns of three sympatric killifish. Copeia 1978:383–389
Barsby T, Kicklighter CE, Hay ME, Sullards MC, Kubanek J (2003) Defensive 2-alkylpyrrole sulfamates from the marine annelid Cirriformia tentaculata. J Nat Prod 66:1110–1112
Bolser R, Hay M (1996) Are tropical plants better defended? Palatability and defenses of temperate vs. tropical seaweeds. Ecology 77:2269–2286
Brusca RC, Brusca GJ (1990) Invertebrates. Sinauer Associates, Inc., Sunderland, MA
Bryan P, McClintock J, Hopkins T (1997) Structural and chemical defenses of echinoderms from the northern Gulf of Mexico. J Exp Mar Biol Ecol 210:173–186
Burns E, Ifrach I, Carmeli S, Pawlik JR, Ilan M (2003) Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical defense. Mar Ecol Prog Ser 252:105–114
Cronin G, Hay ME (1996) Within-plant variation in seaweed palatability and chemical defenses: optimal defense theory versus the growth-differentiation balance hypothesis. Oecologia 105:361–368
Cruz-Rivera E, Hay ME (2003) Prey nutritional quality interacts with chemical defenses to affect consumer feeding and fitness. Ecol Monogr 73:483–506
Duffy J, Paul V (1992) Prey nutritional quality and the effectiveness of chemical defenses against tropical reef fishes. Oecologia 90:333–339
Dunlap M, Pawlik JR (1996) Video monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges. Mar Biol 126:117–123
Fauchald K (1977) The Polychaete worms: definitions and keys to the orders, families and genera. Nat Hist Mus Los Angel County Sci Ser 28:1–190
Fauchald K, Jumars PA (1979) The diet of worms: a study of polychaete feeding guilds. Oceanogr Mar Biol Annu Rev 17:193–284
Feddern HA (1965) The spawning, growth, and general behavior of the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae). Bull Mar Sci 15:896–941
Gaston GR, Slattery M (2002) Ecological function of chemical deterrents in a tropical polychaete, Eupolymnia crassicornis (Annelida, Terebellidae), in Belize. Bull Mar Sci 70:891–897
Gerhart D, Rittschof D, Mayo S (1988) Chemical ecology and the search for marine antifoulants. J Chem Ecol 14:1905–1917
Hay ME (1984) Predictable spatial escapes from herbivory: how do these affect the evolution of herbivore resistance in tropical marine communities? Oecologia 64:396–407
Hay ME (1985) Spatial patterns of herbivore impact and their importance in maintaining algal species richness. In: Proc 5th Int Coral Reef Congress, 27 May–1 June 1985, Tahiti, vol 4, pp 29–34
Hay ME (1986) Associational plant defenses and the maintenance of species diversity: turning competitors into accomplices. Am Nat 128:617–641
Hay ME (1996) Marine chemical ecology: What is known and what is next? J Exp Mar Biol Ecol 200:103–134
Hay ME (1997) The ecology and evolution of seaweed–herbivore interactions on coral reefs. Coral Reefs 16:S67–S76
Hay ME, Fenical W (1988) Marine plant–herbivore interactions—the ecology of chemical defense. Annu Rev Ecol Syst 19:111–145
Hay ME, Kappel QE, Fenical W (1994) Synergisms in plant defenses against herbivores: interactions of chemistry, calcification and plant quality. Ecology 75:1714–1726
Hay ME, Stachowicz JJ, Cruz-Rivera E, Bullard S, Deal MS, Lindquist N (1998a) Bioassays with marine and freshwater macroorganisms. In: Haynes KF, Millar JG (eds) Methods in chemical ecology. Chapman and Hall, New York, pp 39–141
Hay ME, Paul VJ, Lewis SM, Gustafson K, Tucker J, Trindell RN (1988b) Can tropical seaweeds reduce herbivory by growing at night? Diel patterns of growth, nitrogen content, herbivory, and chemical versus morphological defenses. Oecologia 75:233–245
Heinrich B (1993) How avian predators constrain caterpillar foraging. In: Stamp NE, Case TM (eds) In Caterpillars: ecological and evolutionary constraints on foraging. Chapman and Hall, New York, pp 224–247
Hershey AE (1987) Tubes and foraging behavior in larval Chironomidae: implications for predator avoidance. Oecologia 73:236–241
Hill MS, Lopez NA, Young KA (2005) Anti-predator defenses in western North Atlantic sponges with evidence of enhanced defense through interactions between spicules and chemicals. Mar Ecol Prog Ser 291:93–102
Hoogland R, Morris D, Tinbergen N (1957) The spines of sticklebacks (Gasterosteus and Pygosteus) as a means of defence against predators (Perca and Esox). Behaviour 10:205–237
Hsueh P, McClintock JB,Hopkins TS (1992) Comparative study of the diets of the blue crabs Callinectes similis and C. sapidus from a mud-bottom habitat in Mobile Bay, Alabama. J Crust Biol 12:615–619
Jones AC, Blum JE, Pawlik JR (2005) Testing for defensive synergy in Caribbean sponges: bad taste or glass spicules? J Exp Mar Biol Ecol 322:667–687
Kats LB, Petranka JW, Sih A (1988) Antipredator defenses and persistence of amphibian larvae with fishes. Ecology 69:1865–1870
Kicklighter CE, Hay ME (2006) Integrating prey defensive traits: contrasts of marine worms from temperate and tropical habitats. Ecol Monogr 76:195–215
Kicklighter CE, Kubanek J, Barsby T, Hay ME (2003) Palatability and defense of some tropical infaunal worms: alkylpyrrole sulfamates as deterrents to fish feeding. Mar Ecol Prog Ser 263:299–306
Kicklighter CE, Fisher CR, Hay ME (2004) Chemical defense of hydrothermal vent and hydrocarbon seep organisms: a preliminary assessment using shallow-water consumers. Mar Ecol Prog Ser 275:11–19
Krause J, Cheng DJS, Kirkman E, Ruxton GD (2000) Species-specific patterns of refuge use in fish: the role of metabolic expenditure and body length. Behaviour 137:1113–1127
Kupchan SM, Britton RW, Lacadie JA, Ziegler MF, Sigel CW (1975) The isolation and structural elucidation of bruceantin and bruceantinol. J Org Chem 40:648–654
Lindquist N, Hay ME (1995) Can small rare prey be chemically defended—the case for marine larvae. Ecology 76:1347–1358
Lindquist N, Hay ME (1996) Palatability and chemical defense of marine larvae. Ecol Monogr 66:431–450
Long JD, Hay ME (2005) Nudibranch chemical defense creates learned aversions in fishes. Mar Ecol Prog Ser 307:199–208
Lubchenco J, Gaines S (1981) A unified approach to marine plant–herbivore interactions: 1. Populations and communities. Annu Rev Ecol Syst 12:405–437
Mahon AR, Amsler CD, McClintock JB, Amsler MO, Baker BJ (2003) Tissue-specific palatability and chemical defenses against macropredators and pathogens in the common articulate brachiopod Liothyrella uva from the Antarctic Peninsula. J Exp Mar Biol Ecol 290:197–210
Matilla J (1997) The importance of shelter, disturbance and prey interactions for predation rates of tube-building polychaetes (Pygospio elegans Claparede) and free-living tubificid oligochaetes. J Exp Mar Biol Ecol 218:215–228
McClintock JB, Baker BJ (2001) Marine chemical ecology. CRC Press, Boca Raton, FL
Menge BA, Lubchenco J (1981) Community organization in temperate and tropical rocky intertidal habitats: prey refuges in relation to consumer pressure gradients. Ecol Monogr 51:429–450
Miller M, Hay M (1998) Effects of fish predation and seaweed competition on the survival and growth of corals. Oecologia 113:231–238
Paul VJ, van Alstyne K (1988) Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimedaceae, Chlorophyta). Coral Reefs 6:263–269
Paul VJ, Hay ME (1986) Seaweed susceptibility to herbivory: chemical and morphological correlates. Mar Ecol Prog Ser 33:255–264
Paul VJ, Puglisi MP, Ritson-Williams R (2006) Marine chemical ecology. Nat Prod Rep 23:153–180
Pavia H, Toth GB, Aberg P (2002) Optimal defense theory: elasticity analysis as a tool to predict intraplant variation in defenses. Ecology 83:891–897
Pawlik JR, Chanas B, Toonen RJ, Fenical W (1995) Defenses of Caribbean sponges against predatory reef fish. I. Chemical deterrency. Mar Ecol Prog Ser 127:183–194
Pennings S, Paul V (1992) Effect of plant toughness, calcification, and chemistry on herbivory by Dolabella auricularia. Ecology 73:1606–1619
Pennings SC, Paul VJ, Dunbar DC, Hamann MT, Lumbang WA, Novack B, Jacobs RS (1999) Unpalatable compounds in the marine gastropod Dolabella auricularia: distribution and effect of diet. J Chem Ecol 25:735–755
Pennings SC, Siska EL, Bertness MD (2001) Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology 82:1344–1359
Pisut DP, Pawlik JR (2002) Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? J Exp Mar Biol Ecol 270:203–214
Ramachandran R, Khan ZR (1991) Mechanisms of resistance in wild-rice Oryza brachyantha to rice Leaffolder Cnaphalocrocis medinalis (Guenee) (Lepidoptera, Pyralidae). J Chem Ecol 17:41–65
Robinson BA (2000) Habitat heterogeneity and tube-dwelling behavior of larval Chironomidae: implications for prey vulnerability. J Fresh Ecol 15:363–370
Rosenthal GA, Berenbaum MR (1992) Herbivores: their interactions with secondary plant metabolites, 2nd edn. Academic, San Diego, CA
Siska EL, Pennings SC, Buck RL, Hanisak MD (2002) Latitudinal variation in palatability of salt-marsh plants: which traits are responsible? Ecology 83:3369–3381
Stachowicz JJ (2001) Chemical ecology of mobile benthic invertebrates: predators and prey, allies and competitors. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC Press, Boca Raton, FL, pp 157–194
Stachowicz JJ, Hay ME (1999) Mutualism and coral persistence: the role of herbivore resistance to algal chemical defense. Ecology 80:2085–2101
Stachowicz JJ, Lindquist N (2000) Hydroid defenses against predators: the importance of secondary metabolites versus nematocysts. Oecologia 124:280–288
Taylor RB, Sotka E, Hay ME (2002) Tissue specific induction of herbivore resistance: seaweed response to amphipod grazing. Oecologia 132:68–76
Taylor RB, Lindquist N, Kubanek J, Hay ME (2003) Intraspecific variance in palatability and defensive chemistry of brown seaweeds: effects on herbivore fitness. Oecologia 136:412–423
Twigg LE, Socha LV (1996) Physical versus chemical defence mechanisms in toxic Gastrolobium. Oecologia 108:21–28
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Van Alstyne K, Paul V (1992) Chemical and structural defenses in the sea fan Gorgonia ventalina: effects against generalist and specialist predators. Coral Reefs 11:155–159
Vermeij GJ (1978) Biogeography and adaptation. Harvard University Press, Cambridge, MA
Wulff JL (1997) Parrotfish predation on cryptic sponges of Caribbean coral reefs. Mar Biol 129:41–52
Zar J (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Acknowledgments
We thank T. Barsby, D. Burkepile, A. Castilla, Z. Hallinan, T. Henkel, A. Hollebone, J. Long, W. O’Neal, D. Pisut, and K. Whalen for help with worm collections. We thank D. Fields and A. Prusak for help with video analysis. We are grateful to N. Lindquist for providing laboratory space at the UNC Institute of Marine Sciences and to the staff of the National Undersea Research Center, the Smithsonian Tropical Research Institute, Bocas del Toro station, and the crew of the Seaward Johnson I for their cooperation. Steve Kohler and two anonymous reviewers improved the manuscript. Funding for this project was provided by NSF predoctoral and IGERT fellowships and a STRI short-term fellowship to C.E.K. and by the Harry and Linda Teasley Endowment to the Georgia Institute of Technology. This research was conducted in accordance with the Georgia Institute of Technology Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Rights and permissions
About this article
Cite this article
Kicklighter, C.E., Hay, M.E. To avoid or deter: interactions among defensive and escape strategies in sabellid worms. Oecologia 151, 161–173 (2007). https://doi.org/10.1007/s00442-006-0567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0567-0