Abstract
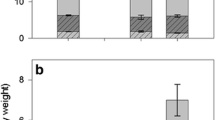
Diapause allows insects to temporally avoid conditions that are unfavorable for development and reproduction. However, diapause may incur a cost in the form of reduced metabolic energy reserves, reduced potential fecundity, and missed reproductive opportunities. This study investigated a hitherto ignored consequence of diapause: trade-offs involving sequestered chemical defense. We examined the aristolochic acid defenses of diapausing and non-diapausing pipevine swallowtail butterflies, Battus philenor. Pipevine swallowtail larvae acquire these chemical defenses from their host plants. Butterflies that emerge following pupal diapause have significantly less fat, a female fitness correlate, compared to those that do not diapause. However, butterflies emerging from diapaused pupae are more chemically defended compared to those that have not undergone diapause. Furthermore, non-diapausing butterflies are confronted with older, lower quality host plants on which to oviposit. Thus, a trade-off exists where butterflies may have greater energy reserves at the cost of less chemical defense and sub-optimal food resources for their larvae, or have substantially less energetic reserves with the benefit of greater chemical defense and plentiful larval food resources.


Similar content being viewed by others
References
Alonso-Mejía A, Brower LP (1994) From model to mimic - age-dependent unpalatability in monarch butterflies. Experientia 50:176–181
Boggs CL (1997) Reproductive allocation from reserves and income in butterfly species with differing adult diets. Ecology 78:181–191
Brower JVZ (1958) Experimental studies of mimicry in some North American butterflies. Part II. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 12:123–136
Chen ZL, Zhu DY (1987) Aristolochia alkaloids. In: Brossi A (ed) The alkaloids: chemistry and pharmacology. Academic, San Diego, pp 29–65
Clancy KM, Price PW (1987) Rapid herbivore growth enhances enemy attack: sublethal plant defenses remain a paradox. Ecology 68:733–737
Codella SG Jr, Lederhouse RC (1989) Intersexual comparison of mimetic protection in the black swallowtail butterfly, Papilio polyxenes: experiments with captive blue jay predators. Evolution 43:410–420
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada (Terrestrial Arthropods), Ottawa
Danks HV (1994) Insect life-cycle polymorphism: current ideas and future prospects. In: Danks HV (ed) Insect life-cycle polymorphism. Kluwer, Dordrecht, pp 349–365
Ellers J, van Alphen JJM (2002) A trade-off between diapause duration and fitness in female parasitoids. Ecol Entomol 27:279–284
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects, vol 10. Plenum, New York, pp 1–40
Fordyce JA (2001) The lethal plant defense paradox remains: inducible host-plant aristolochic acids and the growth and defense of the pipevine swallowtail. Entomol Exp Appl 100:339–346
Fordyce JA (2003) Aggregative feeding of pipevine swallowtall larvae enhances hostplant suitability. Oecologia 135:250–257
Fordyce JA (2006) Interactions between clutches affect a benefit of group feeding for pipevine swallowtail larvae. Ecol Entomol 31:75–83
Fordyce JA, Nice CC (2004) Geographic variation in clutch size and a realized benefit of aggregative feeding. Evolution 58:447–450
Fordyce JA, Shapiro AM (2003) Another perspective on the slow-growth/high-mortality hypothesis: chilling effects on swallowtail larvae. Ecology 84:263–268
Fordyce JA, Marion ZH, Shapiro AM (2005) Phenological variation in chemical defense of the pipevine swallowtail, Battus philenor. J Chem Ecol 31:2835–2846
Hopper KR (1999) Risk-spreading and bet-hedging in insect populations biology. Annu Rev Entomol 44:535–560
Ishihara M, Shimada M (1995) Trade-off in allocation of metabolic reserves - effects of diapause on egg-production and adult longevity in a multivotine bruchid, Kytorhinus-sharpianus. Funct Ecol 9:618–624
Jeffords MR, Sternburg JG, Waldbauer GP (1979) Batesian mimicry: field demonstration of the survival value of pipevine swallowtail and monarch color patterns. Evolution 33:275–286
Karlsson B (1995) Resource allocation and mating systems in butterflies. Evolution 49:955–961
Lill JT (2001) Selection on herbivore life-history traits by the first and third trophic levels: the devil and the deep blue sea revisited. Evolution 55:2236–2247
Moranz R, Brower LP (1998) Geographic and temporal variation of cardenolide-based chemical defenses of queen butterfly (Danaus gilippus) in northern Florida. J Chem Ecol 24:905–932
Mousseau TA, Roff DA (1989) Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43:1483–1496
Nice CC, Fordyce JA (2006) How caterpillars avoid overheating: behavioral and phenotypic plasticity of pipevine swallowtail larvae. Oecologia 146:541–548
Nylin S, Gotthard K (1998) Plasticity in life-history traits. Annu Rev Entomol 43:63–83
Pfeifer HW (1966) Revision of the North and Central American hexandrous species of Aristolochia (Aristolochiaceae). Ann Mo Bot Gard 53:115–196
Pfeifer HW (1970) A taxonomic revision of the pentandrous species of Aristolochia. The University of Connecticut Publication Series, 134
Racheli T, Pariset L (1992) II genere Battus tassonomia e storia naturale. Fragm Entomol (Suppl) 23:1–163
Rausher MD (1986) Competition, frequency-dependent selection, and diapause in Battus philenor butterflies. Fla Entomol 69:63–78
Ritland DB (1994) Variation in palatability of Queen butterflies (Danaus gilippus) and implications regarding mimicry. Ecology 75:732–746
Saunders DS (2000) Larval diapause duration and fat metabolism in three geographical strains of the blow fly, Calliphora vicina. J Insect Physiol 46:509–517
Scott JA (1986) The butterflies of North America. Stanford University Press, Stanford
Sime KR, Feeny PP, Haribal MM (2000) Sequestration of aristolochic acids by the pipevine swallowtail, Battus philenor (L.): evidence and ecological implications. Chemoecology 10:169–178
Sims SR, Shapiro AM (1983a) Pupal color dimorphism in California Battus philenor (L.) (Papilionidae): mortality factors and selective advantage. J Lepid Soc 37:236–243
Sims SR, Shapiro AM (1983b) Pupal diapause in Battus philenor (Lepidoptera: Papilionidae). Ann Entomol Soc Am 76:407–412
Soula B, Menu F (2003) Variability in diapause duration in the chestnut weevil: mixed ESS, genetic polymorphism or bet-hedging? Oikos 100:574–580
Tauber CA, Tauber MJ (1992) Phenotypic plasticity in Chrysoperla: genetic variation in the sensory mechanism and in correlated reproductive traits. Evolution 46:1754–1773
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York
Urzúa A, Priestap H (1985) Aristolochic acids from Battus polydamas. Biochem Syst Ecol 13:169–170
Urzúa A, Rodriguez R, Cassesl B (1987) Fate of ingested aristolochic acids in Battus Archidamus. Biochem Syst Ecol 15:687–690
Wiklund C, Wickman PO, Nylin S (1992) A sex difference in the propensity to enter direct/diapause development - a result of selection for protandry. Evolution 46:519–528
Williams IS (1999) Slow-growth, high-mortality - A general hypothesis, or is it? Ecol Entomol 24:490–495
Acknowledgements
We thank K. Fordyce and M. Spencer for their assistance with butterfly rearing and Z. Marion for help with chemical analysis. Thanks to B. Fitzpatrick for help with the program R. This manuscript was improved by the helpful comments of M. Boercker, L. McDonald and two anonymous reviewers. Thanks to V. Boucher and D. Tolson of the UCNRS for access to Stebbins Cold Canyon Reserve and Texas State University for access to Freeman Ranch. This study was supported by the University of Tennessee, Texas State University, Center for Population Biology (UC-Davis), and the U.S. National Science Foundation (DEB-9306721) to A.M.S. and (DBI-0317483) to A.M.S. and James Quinn and (DEB-0614223) to J.A.F.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Richard Karban
Rights and permissions
About this article
Cite this article
Fordyce, J.A., Nice, C.C. & Shapiro, A.M. A novel trade-off of insect diapause affecting a sequestered chemical defense. Oecologia 149, 101–106 (2006). https://doi.org/10.1007/s00442-006-0428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0428-x