Abstract
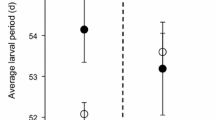
Despite ecologists increasingly recognizing pathogens as playing significant roles in community dynamics, few experimental studies have quantified patterns of disease impacts on natural systems. Amphibians are experiencing population declines, and a fungal pathogen (Batrachochytrium dendrobatidis; Chytridiomycota) is a suspected causal agent in many declines. We studied the effects of a pathogenic fungus on community interactions between the gray treefrog, Hyla chrysoscelis, and eastern newts, Notophthalmus viridescens. Recent studies have characterized chytridiomycosis as an emerging infectious disease, whose suspected rapid range expansion and widespread occurrence pose a significant risk for amphibian populations worldwide. We reared larvae in outdoor polyethylene experimental tanks and tested the effects of initial larval density, predator presence, and fungal exposure on Hyla recruitment and predator-prey interactions between Hyla and Notophthalmus. Newts reduced treefrog survival, and high intraspecific density decreased metamorphic body mass independent of B. dendrobatidis. The presence of fungi reduced treefrog body mass at metamorphosis by 34%, but had no significant main effect on survival or larval period length. B. dendrobatidis differentially affected larval development in the presence of predators; Hyla developed slower when reared with the pathogen, but only when newts were present. This significant predator-by-pathogen interaction suggests that the impact of chytridiomycosis on larval amphibians may be exacerbated in complex communities. Our data suggest that B. dendrobatidis effects on host life history may be complex and indirect. Direct measurements of the community-level effects of pathogens offer an important opportunity to understand a significant threat to global biodiversity—declining amphibian populations.



Similar content being viewed by others
References
Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
American Public Health Association, American Water Works Association, and the Water Pollution Control Federation (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, New York
Beebee TJ (1995) Tadpole growth: is there an interference effect in nature? Herpetol J 5:204–205
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036
Blaustein AR, Hoffman PD, Hokit DG, Kiesecker JM, Walls SD, Hays JB (1994) UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proc Natl Acad Sci USA 91:1791–1795
Boone MD, Bridges CM (1999) The effect of temperature on the potency of carbaryl for tadpoles of the green frog, Rana clamitans. Environ Toxicol Chem 18:1482–1484
Boone MD, Semlitsch RD (2001) Interactions of an insecticide with larval density and predation in experimental amphibian communities. Conserv Biol 15:228–238
Bosch J, Martínez-Solano I, García-París M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv 97:331–337
Bradley GA, Rosen PC, Sredl MJ, Jones TR, Longcore JE (2002) Chytridiomycosis in native Arizona frogs. J Wild Dis 38:206–212
Bronmark C, Rundle SD, Erlandsson J (1991) Interactions between freshwater snails and tadpoles: competition and facilitation. Oecologia 87:8–18
Carey C (2000) Infectious disease and worldwide declines of amphibian populations, with comments on emerging diseases in coral reef organisms and in humans. Environ Health Perspect 108:143–150
Collins JP, Brunner JL, Miera V, Parris MJ, Schock DM, Storfer A (2003) Ecology and evolution of infectious disease. In: Semlitsch RD (ed) Amphibian conservation. Smithsonian Institution Press, Washington, pp 137–151
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Div Distrib 9:141–150
Davidson EW, Parris MJ, Collins JP, Longcore JE, Pessier AP, Brunner J (2003) Pathogenicity and transmission of chytridiomycosis in tiger salamanders (Ambystoma tigrinum). Copeia 2003:601–607
Denver R, Mirhadi N, Phillips M (1998) Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 79:1859–1872
Dobson AP, Crawley M (1994) Pathogens and the structure of plant communities. Trends Ecol Evol 9:393–398
Dunn AM, Dick JT (1998) Parasitism and epibiosis in native and non-native gammarids in freshwater in Ireland. Ecography 21:593–598
Fellers GM, Green DE, Longcore JE (2001) Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa). Copeia 2001:945–953
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Griffiths RA (1995) Determining competition mechanisms in tadpole assemblages. Herpetol J 5:208–210
Hopkins S, Channing A (2002) Chytridiomycosis in Northern and Western Cape frog populations, South Africa. Froglog 54:1–2
Kiesecker JM, Blaustein AR (1999) Pathogen reverses competition between larval amphibians. Ecology 80:2442–2448
Kiesecker JM, Skelly DK (2001) Effects of disease and pond drying on gray tree frog growth, development, and survival. Ecology 82:1956–1963
Kohler SL, Wiley MJ (1997) Pathogen outbreaks reveal large-scale effects of competition in stream communities. Ecology 78:2164–2176
Lips KR (1999) Mass mortality and population declines of anurans at an upland site in western Panama. Conserv Biol 13:117–125
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
Mara BD (1974) Bacteriology for sanitary engineers. Longman, New York
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 12:320–325
Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130:730–757
Mitchell JE, Green DE (2002) Chytridiomycosis in two species of ranid frogs in the southeastern United States. In: Joint meeting of the American Society of Ichthyologists and Herpetologists, Herpetologists’ League, and the Society for the Study of Amphibians and Reptiles, 4–8 July 2002, Kansas City
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Morin PJ (1983) Predation, competition, and the composition of larval anuran guilds. Ecol Mongr 53:119–138
Newman R (1988) Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution 42:774–783
Nichols DK, Lamirande EW, Pessier AP, Longcore JE (2001) Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wild Dis 37:1–11
Parris MJ (1999) Hybridization in leopard frogs (Rana pipiens complex): larval fitness components in single-genotype populations and mixtures. Evolution 53:1872–1883
Parris MJ (2004) Hybrid response to pathogen infection in interspecific crosses between two amphibian species (Anura: Ranidae). Evol Ecol Res 6:457–471
Parris MJ, Baud DR (2004) Interactive effects of a heavy metal and chytridiomycosis on Gray Treefrog larvae (Hyla chrysoscelis). Copeia 2004:343–349
Parris MJ, Semlitsch RD (1998) Asymmetric competition in larval amphibian communities: conservation implications for the northern crawfish frog, Rana areolata circulosa. Oecologia 116:219–226
Parris MJ, Laird CW, Semlitsch RD (2001) Differential predation on experimental populations of parental and hybrid leopard frog (Rana blairi and Rana sphenocephala) larvae. J Herpetol 35:479–485
Pessier AP, Nichols DK, Longcore JE, Fuller MS (1999) Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J Vet Diagnost Invest 11:194–199
Petranka JW (1989) Chemical interference competition in tadpoles: does it occur outside laboratory aquaria? Copeia 1989:921–930
Price PW, Westoby M, Rice B (1988) Parasite-mediated competition: some predictions and tests. Am Nat 131:544–555
Relyea RA (2001) The lasting effects of adaptive plasticity: predator-induced tadpoles become long-legged frogs. Ecology 82:1947–1955
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci USA 98:2491–2496
Rollins-Smith LA, Doersam JK, Longcore JE, Taylor SK, Shamblin JC, Carey C, Zasloff MA (2002) Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev Comp Immunol 26:63–72
SAS Institute (1990) SAS/STAT user’s guide. Vers 6, 4th edn. SAS Institute, Cary, N.C.
Semlitsch RD, Scott DE, Pechmann JHK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Skelly DK (1992) Field evidence for a cost of behavioral antipredator response in a larval amphibian. Ecology 73:704–708
Skelly DK (1997) Tadpole communities. Am Sci 85:36–45
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Tiedje JM, Colwell RK, Grossman YL, Hodson RE, Lenski RE, Mack RN, Regal PJ (1989) The planned introduction of genetically engineered organisms: ecological considerations and recommendations. Ecology 70:298–315
Travis J (1984) Anuran size at metamorphosis: experimental test of a model based on intraspecific competition. Ecology 65:1155–1160
Washburn JO, Mercer DR, Anderson JR (1991) Regulatory role of parasites: impact on host population shifts with resource availability. Science 253:185–188
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 138:319–341
Werner EE, Anholt BR (1996) Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology 77:157–169
Werner EE, Glennemeier KS (1999) Influence of forest canopy cover on the breeding pond distributions of several amphibian species. Copeia 1999:1–12
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Woodward BD (1983) Predator-prey interactions and breeding pond use of temporary pond species in a desert anuran community. Ecology 64:1549–1555
Acknowledgements
We thank J. Longcore for supplying the B. dendrobatidis isolates. We are grateful for the support J. Grubaugh provided throughout the work, and we thank S. Kynerd for helping in construction of the cages. D. VanVickle provided a much-appreciated watchful eye on the experimental tanks. J. Collins and M. Ferkin provided helpful comments on the manuscript. Experiments were conducted in accordance with the goals and regulations of the University of Memphis Edward J. Meeman Biological Field Station. This research was partially supported by the University of Memphis Department of Biology and NSF Integrated Research Challenges in Environmental Biology grant IBN 9977063.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parris, M.J., Beaudoin, J.G. Chytridiomycosis impacts predator-prey interactions in larval amphibian communities. Oecologia 140, 626–632 (2004). https://doi.org/10.1007/s00442-004-1631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1631-2