Abstract
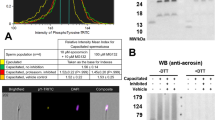
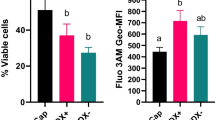
Protein ubiquitination is a stable, reversible post-translational modification, targeting proteins for degradation/recycling by the 26S proteasome in a well-characterized enzymatic cascade. Studies have revealed the role of UPS in the regulation of fertilization, including sperm–zona pellucida interactions and the early event of sperm capacitation. The present study investigates the changes in proteasome compartmentalization, subunit composition and post-translational modifications during in vitro capacitation of fresh boar spermatozoa. We observed capacitation-dependent shedding of both 20S core and 19S regulatory particles from the acrosome that was associated with decreased plasma membrane integrity, independent of proteasomal inhibition. Subunits PSMA1–7 of the 20S core did not appear to undergo post-translational modifications during capacitation, based on invariant molecular masses before and after capacitation; however, we observed multiple PSMD4 forms of 19S regulatory particles (50, 53, 70, 115–140, 160 and >176 kDa) sequentially released from spermatozoa. PSMD4 subunit was found to be post-translationally modified during the course of capacitation, resulting in changes of apparent molecular mass, some of which were dependent on proteasomal inhibition. These results show that the sperm proteasomes are being modified during sperm capacitation. Additional studies of individual 26S proteasome subunits will be required to elucidate these modifications and to understand how UPS modulates sperm capacitation.




Similar content being viewed by others
References
Austin CR (1952) The capacitation of the mammalian sperm. Nature 170:326
Bose S, Stratford FL, Broadfoot KI, Mason GG, Rivett AJ (2004) Phosphorylation of 20S proteasome alpha subunit C8 (alpha7) stabilizes the 26S proteasome and plays a role in the regulation of proteasome complexes by gamma-interferon. Biochem J 378:177–184
Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, Gygi SP, Finley D (2006) Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127:1401–1413
Deveraux Q, Ustrell V, Pickart C, Rechsteiner M (1994) A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem 269:7059–7061
Diaz ES, Kong M, Morales P (2007) Effect of fibronectin on proteasome activity, acrosome reaction, tyrosine phosphorylation and intracellular calcium concentrations of human sperm. Hum Reprod 22:1420–1430
Feng Y, Longo DL, Ferris DK (2001) Polo-like kinase interacts with proteasomes and regulates their activity. Cell Growth Differ 12:29–37
Fernandez Murray P, Pardo PS, Zelada AM, Passeron S (2002) In vivo and in vitro phosphorylation of Candida albicans 20S proteasome. Arch Biochem Biophys 404:116–125
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Chang MC (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168:697–698
Isasa M, Katz EJ, Kim W, Yugo V, Gonzalez S, Kirkpatrick DS, Thomson TM, Finley D, Gygi SP, Crosas B (2010) Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome. Mol Cell 38:733–745
Jacobson AD, Macfadden A, Wu Z, Peng J, Liu CW (2014) Autoregulation of the 26S proteasome by in situ ubiquitination. Mol Biol Cell 25:1824–1835
Kerns K, Morales P, Sutovsky P (2016) Regulation of sperm capacitation by the 26S proteasome: an emerging new paradigm in spermatology. Biol Reprod 94:117
Kong M, Diaz ES, Morales P (2009) Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol Reprod 80:1026–1035
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lipinszki Z, Kovacs L, Deak P, Udvardy A (2012) Ubiquitylation of drosophila p54/Rpn10/S5a regulates its interaction with the UBA-UBL polyubiquitin receptors. Biochemistry 51:2461–2470
Miles EL, O’gorman C, Zhao J, Samuel M, Walters E, Yi YJ, Sutovsky M, Prather RS, Wells KD, Sutovsky P (2013) Transgenic pig carrying green fluorescent proteasomes. Proc Natl Acad Sci U S A 110:6334–6339
Mochida K, Tres LL, Kierszenbaum AL (2000) Structural features of the 26S proteasome complex isolated from rat testis and sperm tail. Mol Reprod Dev 57:176–184
Morales P, Diaz ES, Kong M (2007) Proteasome activity and its relationship with protein phosphorylation during capacitation and acrosome reaction in human spermatozoa. Soc Reprod Fertil Suppl 65:269–273
Sanchez R, Deppe M, Schulz M, Bravo P, Villegas J, Morales P, Risopatron J (2011) Participation of the sperm proteasome during in vitro fertilisation and the acrosome reaction in cattle. Andrologia 43:114–120
Sasanami T, Sugiura K, Tokumoto T, Yoshizaki N, Dohra H, Nishio S, Mizushima S, Hiyama G, Matsuda T (2012) Sperm proteasome degrades egg envelope glycoprotein ZP1 during fertilization of Japanese quail (Coturnix Japonica). Reproduction 144:423–431
Satoh K, Sasajima H, Nyoumura KI, Yokosawa H, Sawada H (2001) Assembly of the 26S proteasome is regulated by phosphorylation of the p45/Rpt6 ATPase subunit. Biochemistry 40:314–319
Sawada H, Pinto MR, De Santis R (1998) Participation of sperm proteasome in fertilization of the phlebobranch ascidian Ciona Intestinalis. Mol Reprod Dev 50:493–498
Sutovsky P (2011) Sperm proteasome and fertilization. Reproduction 142:1–14
Sutovsky P, Manandhar G, McCauley TC, Caamano JN, Sutovsky M, Thompson WE, Day BN (2004) Proteasomal interference prevents zona pellucida penetration and fertilization in mammals. Biol Reprod 71:1625–1637
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, vol 1. Raven, New York, pp 189–317
Yi YJ, Manandhar G, Oko RJ, Breed WG, Sutovsky P (2007) Mechanism of sperm-zona pellucida penetration during mammalian fertilization: 26S proteasome as a candidate egg coat lysin. Soc Reprod Fertil Suppl 63:385–408
Yi YJ, Manandhar G, Sutovsky M, Jonakova V, Park CS, Sutovsky P (2010a) Inhibition of 19S proteasomal regulatory complex subunit PSMD8 increases polyspermy during porcine fertilization in vitro. J Reprod Immunol 84:154–163
Yi YJ, Manandhar G, Sutovsky M, Zimmerman SW, Jonakova V, Van Leeuwen FW, Oko R, Park CS, Sutovsky P (2010b) Interference with the 19S proteasomal regulatory complex subunit PSMD4 on the sperm surface inhibits sperm-zona pellucida penetration during porcine fertilization. Cell Tissue Res 341:325–340
Yi YJ, Park CS, Kim ES, Song ES, Jeong JH, Sutovsky P (2009) Sperm-surface ATP in boar spermatozoa is required for fertilization: relevance to sperm proteasomal function. Syst Biol Reprod Med 55:85–96
Yi YJ, Zimmerman SW, Manandhar G, Odhiambo JF, Kennedy C, Jonakova V, Manaskova-Postlerova P, Sutovsky M, Park CS, Sutovsky P (2012) Ubiquitin-activating enzyme (UBA1) is required for sperm capacitation, acrosomal exocytosis and sperm-egg coat penetration during porcine fertilization. Int J Androl 35:196–210
Yokota N, Kataoka Y, Hashii N, Kawasaki N, Sawada H (2011) Sperm-specific C-terminal processing of the proteasome PSMA1/alpha6 subunit. Biochem Biophys Res Commun 410:809–815
Yokota N, Sawada H (2007) Sperm proteasomes are responsible for the acrosome reaction and sperm penetration of the vitelline envelope during fertilization of the sea urchin Pseudocentrotus Depressus. Dev Biol 308:222–231
Zimmerman S, Sutovsky P (2009) The sperm proteasome during sperm capacitation and fertilization. J Reprod Immunol 83:19–25
Zimmerman SW, Manandhar G, Yi YJ, Gupta SK, Sutovsky M, Odhiambo JF, Powell MD, Miller DJ, Sutovsky P (2011) Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS ONE 6:e17256
Acknowledgements
We thank the staff of the National Swine Research Resource Center, University of Missouri, for boar semen collections and Ms. Kathy Craighead for editorial and administrative assistance. This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2015-67015-23231 from the USDA NIFA (PS), seed funding from the F21C Program of the University of Missouri (PS), project BIOCEV CZ.1.05/1.1.00/02.0109 from the ERDF (MZ), the CAS (RVO: 86652036) (MZ) and USDA NIFA Graduate Fellowship Award number 2017-67011-26023 (KK).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 643 kb)
Rights and permissions
About this article
Cite this article
Zigo, M., Kerns, K., Sutovsky, M. et al. Modifications of the 26S proteasome during boar sperm capacitation. Cell Tissue Res 372, 591–601 (2018). https://doi.org/10.1007/s00441-017-2786-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2786-6