Abstract
From the moment we are born, every injury to the skin has the potential to form a scar, many of which can impair form and/or function. As such, scar management constitutes a billion-dollar industry. However, effectively promoting scarless wound healing remains an elusive goal. The complex interactions of wound healing contribute to our inability to recapitulate scarless wound repair as it occurs in nature, such as in fetal skin and the oral mucosa. However, many new advances have occurred in recent years, some of which have translated scientific findings from bench to bedside. In vivo lineage tracing has helped establish a variety of novel cellular culprits that may act as key drivers of the fibrotic response. These newly characterized cell populations present further targets for therapeutic intervention, some of which have previously demonstrated promising results in animal models. Here, we discuss several recent studies that identify exciting approaches for diminishing scar formation. Particular attention will also be paid to the canonical Wnt/β-catenin signaling pathway, which plays an important role in both embryogenesis and tissue repair. New insights into the differential effects of Wnt signaling on heterogeneous fibroblast and keratinocyte populations within the skin further demonstrate methods by which wound healing can be re-directed to a more fetal scarless phenotype.

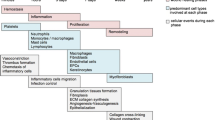
Recent approaches to reducing scar formation. Representation showing novel scientific approaches for decreasing scar formation, including the targeting of pro-fibrotic cell populations based on surface molecule expression (e.g. DPP4+ fibroblasts, ADAM12+ pericytes). Modulation of cellular mechanotransduction pathways are another means to reduce scar formation, both at the molecular level or, macroscopically with dressings designed to offload tension, at cutaneous wound sites (ADAM12 a disintegrin and metalloprotease 12, DPP4 dipeptidyl peptidase-4, FAK focal adhesion kinase)




Similar content being viewed by others
References
Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener M-O, MacDougald OA, Distler O, Schett G, Distler JHW (2012) Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3:735
Amerongen AV, Veerman EC (2002) Saliva—the defender of the oral cavity. Oral Dis 8:12–22
Bastakoty D, Saraswati S, Cates J, Lee E, Nanney LB, Young PP (2015) Inhibition of Wnt/beta-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J 29:4881–4892
Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD (2009) SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5:610–623
Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP (2010) Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg 125:74–88
CDC (2010) Centers for disease control and prevention. National hospital discharge survey: 2010 table. Procedures by selected patient characteristics. Number by procedure category and age. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
Cerqueira MT, Pirraco RP, Marques AP (2015) Stem cells in skin wound healing: are we there yet? Adv Wound Care (New Rochelle) 5:164-175
Cheng J, Yu H, Deng S, Shen G (2010) MicroRNA profiling in mid- and late-gestational fetal skin: implication for scarless wound healing. Tohoku J Exp Med 221:203–209
Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA (2002) Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A 99:6973–6978
Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA (2006) Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J 20:692–701
Clevers H, Loh KM, Nusse R (2014) Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346:1248012
Collins CA, Kretzschmar K, Watt FM (2011) Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development 138:5189–5199
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372
Cullen KA, Hall MJ, Golosinskiy A (2009) Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009:1-25
Desai VD, Hsia HC, Schwarzbauer JE (2014) Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One 9:e86865
Desmouliere A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
de la Roche M, Ibrahim AE, Mieszczanek J, Bienz M (2014) LEF1 and B9L shield beta-catenin from inactivation by Axin, desensitizing colorectal cancer cells to tankyrase inhibitors. Cancer Res 74:1495–1505
de Souza KS, Cantaruti TA, Azevedo GM Jr, Galdino DA, Rodrigues CM, Costa RA, Vaz NM, Carvalho CR (2015) Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Exp Dermatol 24:198–203
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Ding J, Ma Z, Liu H, Kwan P, Iwashina T, Shankowsky HA, Wong D, Tredget EE (2014) The therapeutic potential of a C-X-C chemokine receptor type 4 (CXCR-4) antagonist on hypertrophic scarring in vivo. Wound Repair Regen 22:622–630
Doi H, Kitajima Y, Luo L, Yan C, Tateishi S, Ono Y, Urata Y, Goto S, Mori R, Masuzaki H, Shimokawa I, Hirano A, Li TS (2016) Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep 6:18844
Driskell RR, Watt FM (2015) Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25:92–99
Driskell RR, Clavel C, Rendl M, Watt FM (2011) Hair follicle dermal papilla cells at a glance. J Cell Sci 124:1179–1182
Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281
Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L (2012) Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18:1262–1270
Finkelstein E, Corso PS, Miller TR (2006) The incidence and economic burden of injuries in the United States. Oxford University Press, Oxford
Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC (2004) Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12:485–492
Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, Nace A, Zhang X, Baratono S, Wang F, Ornitz DM, Millar SE, Cotsarelis G (2013) Fgf9 from dermal [gamma][delta] T cells induces hair follicle neogenesis after wounding. Nat Med 19:916–923
Glim JE, Everts V, Niessen FB, Ulrich MM, Beelen RH (2014) Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch Oral Biol 59:1048–1055
Glim JE, Beelen RH, Niessen FB, Everts V, Ulrich MM (2015) The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Arch Oral Biol 60:272–281
Gras C, Ratuszny D, Hadamitzky C, Zhang H, Blasczyk R, Figueiredo C (2015) miR-145 contributes to hypertrophic scarring of the skin by inducing myofibroblast activity. Mol Med 21:296–304
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170:1807–1816
Ho S, Marcal H, Foster LJ (2014) Towards scarless wound healing: a comparison of protein expression between human, adult and foetal fibroblasts. Biomed Res Int 2014:676493
Houschyar KS, Momeni A, Pyles MN, Maan ZN, Whittam AJ, Siemers F (2015) Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 11:95–104
Hu MS, Januszyk M, Hong WX, Walmsley GG, Zielins ER, Atashroo DA, Maan ZN, McArdle A, Takanishi DM Jr, Gurtner GC, Longaker MT, Lorenz HP (2014) Gene expression in fetal murine keratinocytes and fibroblasts. J Surg Res 190:344–357
Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11:1351–1354
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447:316–320
Kieran I, Knock A, Bush J, So K, Metcalfe A, Hobson R, Mason T, O’Kane S, Ferguson M (2013) Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: results of two preclinical and phase II randomized control studies. Wound Repair Regen 21:428–436
Lam AP, Gottardi CJ (2011) Beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol 23:562–567
Larson BJ, Longaker MT, Lorenz HP (2010) Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 126:1172–1180
Lee SH, Kim MY, Kim HY, Lee YM, Kim H, Nam KA, Roh MR, Mindo S, Chung KY, Choi KY (2015) The dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J Exp Med 212:1061–1080
Lee WJ, Park JH, Shin JU, Noh H, Lew DH, Yang WI, Yun CO, Lee KH, Lee JH (2015) Endothelial-to-mesenchymal transition induced by Wnt 3a in keloid pathogenesis. Wound Repair Regen 23:435–442
Li M, Luan F, Zhao Y, Hao H, Liu J, Dong L, Fu X, Han W (2015) Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int Wound J 10.1111/iwj.12551
Lichtenberger BM, Mastrogiannaki M, Watt FM (2016) Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun 7:10537
Lim AF, Weintraub J, Kaplan EN, Januszyk M, Cowley C, McLaughlin P, Beasley B, Gurtner GC, Longaker MT (2014) The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg 133:398–405
Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS (1994) Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 219:65–72
Longaker MT, Rohrich RJ, Greenberg L, Furnas H, Wald R, Bansal V, Seify H, Tran A, Weston J, Korman JM, Chan R, Kaufman D, Dev VR, Mele JA, Januszyk M, Cowley C, McLaughlin P, Beasley B, Gurtner GC (2014) A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg 134:536–546
Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E (1998) Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 111:850–857
Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS (1992) Scarless wound repair: a human fetal skin model. Development 114:253–259
Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK (2001) Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci 42:2490–2495
Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C (2012) Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489:257–262
Mia MM, Bank RA (2015) Paracrine factors of human amniotic fluid-derived mesenchymal stem cells show strong anti-fibrotic properties by inhibiting myofibroblast differentiation and collagen synthesis. J Stem Cell Res Ther 5:282
Monaghan M, Browne S, Schenke-Layland K, Pandit A (2014) A collagen-based scaffold delivering exogenous microrna-29B to modulate extracellular matrix remodeling. Mol Ther 22:786–796
Morris MW Jr, Allukian M 3rd, Herdrich BJ, Caskey RC, Zgheib C, Xu J, Dorsett-Martin W, Mitchell ME, Liechty KW (2014) Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound Repair Regen 22:406–414
Nuschke A (2014) Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 10:29–37
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB (1999) Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277:C1–C19
Rinella L, Marano F, Berta L, Bosco O, Fraccalvieri M, Fortunati N, Frairia R, Catalano MG (2016) Extracorporeal shock waves modulate myofibroblast differentiation of adipose-derived stem cells. Wound Repair Regen 24:275–286
Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT (2015) Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348:aaa2151
Robert R, Meyer W, Bishop S, Rosenberg L, Murphy L, Blakeney P (1999) Disfiguring burn scars and adolescent self-esteem. Burns 25:581–585
Sabapathy V, Sundaram B, Sreelakshmi VM, Mankuzhy P, Kumar S (2014) Human Wharton’s Jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One 9:e93726
Sato M (2006) Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol 86:300–307
Schmidt BA, Horsley V (2013) Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140:1517–1527
Sen CK, Ghatak S (2015) miRNA control of tissue repair and regeneration. Am J Pathol 185:2629–2640
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17:763–771
Sheridan RL, Hinson MI, Liang MH, Nackel AF, Schoenfeld DA, Ryan CM, Mulligan JL, Tompkins RG (2000) Long-term outcome of children surviving massive burns. JAMA 283:69–73
Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, Chen L, Qi S, Liu X, Wang P, Tang J, Xie J (2015) Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther 6:120
Singer AJ, Hollander JE, Quinn JV (1997) Evaluation and management of traumatic lacerations. N Engl J Med 337:1142–1148
Singla DK, Singla RD, Abdelli LS, Glass C (2015) Fibroblast growth factor-9 enhances M2 macrophage differentiation and attenuates adverse cardiac remodeling in the infarcted diabetic heart. PLoS One 10:e0120739
Thielitz A, Vetter RW, Schultze B, Wrenger S, Simeoni L, Ansorge S, Neubert K, Faust J, Lindenlaub P, Gollnick HP, Reinhold D (2008) Inhibitors of dipeptidyl peptidase IV-like activity mediate antifibrotic effects in normal and keloid-derived skin fibroblasts. J Invest Dermatol 128:855–866
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Wang Z, Liu X, Zhang D, Wang X, Zhao F, Zhang T, Wang R, Lin X, Shi P, Pang X (2015) Phenotypic and functional modulation of 20–30 year old dermal fibroblasts by mid- and late-gestational keratinocytes in vitro. Burns 41:1064–1075
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC (2012) Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18:148–152
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Zhao F, Wang Z, Lang H, Liu X, Zhang D, Wang X, Zhang T, Wang R, Shi P, Pang X (2015) Dynamic expression of novel MiRNA candidates and MiRNA-34 family members in early- to mid-gestational fetal keratinocytes contributes to scarless wound healing by targeting the TGF-beta pathway. PLoS One 10:e0126087
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure
The authors have no conflicting financial interests to disclose.
Additional information
This work was supported in part by the California Institute for Regenerative Medicine (CIRM) Clinical Fellow training grant TG2-01159 (to M.S.H.), the Stanford University School of Medicine Transplant and Tissue Engineering Fellowship Award (to M.S.H.), the American Society of Maxillofacial Surgeons (ASMS)/Maxillofacial Surgeons Foundation (MSF) Research Grant Award (to M.S.H., H.P.L., and M.T.L.), the American College of Surgeons Resident Research Scholarship (to C.D.M.), the Howard Hughes Medical Institute (to L.A.B.), NIH grant R01 GM087609 (to H.P.L.), a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (to H.P.L.), the Hagey Laboratory for Pediatric Regenerative Medicine and The Oak Foundation (to H.P.L. and M.T.L.), NIH grant U01 HL099776 (to M.T.L.), and the Gunn/Olivier fund (to M.T.L.).
Rights and permissions
About this article
Cite this article
Leavitt, T., Hu, M.S., Marshall, C.D. et al. Scarless wound healing: finding the right cells and signals. Cell Tissue Res 365, 483–493 (2016). https://doi.org/10.1007/s00441-016-2424-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2424-8