Abstract
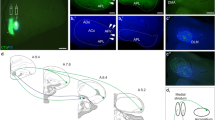
Several studies have shown that L-aspartate (Asp) is present in synaptic vesicles and released exocytotically from presynaptic terminals, possibly by Ca2+-dependent corelease of Asp and L-glutamate (Glu). It has been demonstrated that both excitatory amino acids (EAAs) are released from the rat striatum as part of corticostriatal neurotransmission. The single or colocalized occurrence of Asp and Glu in specific synaptic boutons of the chicken medial striatum/nucl. accumbens has been demonstrated by our group using ultrastructural immunocytochemistry. However, evidence for the presence of EAAs in any specific striatal pathway was only circumstantial. Here, we report on the distribution of Asp and Glu in specific synaptic terminals of the amygdalostriatal pathway, both in rat and chicken brains, combining anterograde tracing with postembedding immunogold labeling of Asp or Glu. Immunoreactivity for Asp and Glu was observed in amygdalofugal terminals with asymmetrical synaptic junctions (morphologically representing excitatory synapses) in both species. The postsynaptic targets were either dendritic spines or small dendrites, whereas axosomatic or axo-axonic connections were not observed. Ultrastructurally, the synaptic terminals immunoreactive for Asp were indistinguishable from those immunoreactive for Glu. The findigs are consistent with an Asp–Glu corelease mechanism, with a distinct synaptic contingent, evolutionarily conserved in the amygdalostriatal pathway.








Similar content being viewed by others
References
Abellan A, Medina L (2008) Expression of cLhx6 and cLhx7/8 suggests a pallido-pedunculo-preoptic origin for the lateral and medial parts of the avian bed nucleus of the stria terminalis. Brain Res Bull 75:299–304
Adam AS, Csillag A (2006) Differential distribution of L-aspartate- and L-glutamate-immunoreactive structures in the arcopallium and medial striatum of the domestic chick (Gallus domesticus). J Comp Neurol 498:266–276
Ambroggi F, Ishikawa A, Fields HL, Nicola SM (2008) Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron 59:648–661
Aoki E, Semba R, Kato K, Kashiwamata S (1987) Purification of specific antibody against aspartate and immunocytochemical localization of aspartergic neurons in the rat brain. Neuroscience 21:755–765
Balint E, Csillag A (2007) Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (Gallus domesticus). Cell Tissue Res 327:221–230
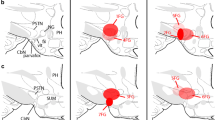
Balint E, Mezey S, Csillag A (2011) Efferent connections of nucleus accumbens subdivisions of the domestic chicken (Gallus domesticus): an anterograde pathway tracing study. J Comp Neurol 519:2922–2953
Baughman RW, Gilbert CD (1980) Aspartate and glutamate as possible neurotransmitters of cells in layer 6 of the visual cortex. Nature 287:848–850
Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF, Renaud B (2002) In vivo temporal sequence of rat striatal glutamate, aspartate and dopamine efflux during apomorphine, nomifensine, NMDA and PDC in situ administration. Neuropharmacology 43:825–835
Bolam JP (1992) Experimental Neuroanatomy - A Practical Approach. Oxford University Press, Oxford
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278
Csillag A, Szekely AD, Stewart MG (1997) Synaptic terminals immunolabelled against glutamate in the lobus parolfactorius of domestic chicks (Gallus domesticus) in relation to afferents from the archistriatum. Brain Res 750:171–179
Csillag A, Balint E, Adam A, Zachar G (2008) The organisation of the basal ganglia in the domestic chick (Gallus domesticus): anatomical localisation of DARPP-32 in relation to glutamate. Brain Res Bull 76:183–191
Davies DC, Csillag A, Szekely AD, Kabai P (1997) Efferent connections of the domestic chick archistriatum: a phaseolus lectin anterograde tracing study. J Comp Neurol 389:679–693
Di Ciano P, Everitt BJ (2001) Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 25:341–360
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci Off J Soc Neurosci 24:7167–7173
Fagg GE, Foster AC (1983) Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience 9:701–719
French SJ, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119:19–31
Fricker-Gates RA, Shin JJ, Tai CC, Catapano LA, Macklis JD (2002) Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J Neurosci Off J Soc Neurosci 22:4045–4056
Girault JA, Barbeito L, Spampinato U, Gozlan H, Glowinski J, Besson MJ (1986) In vivo release of endogenous amino acids from the rat striatum: further evidence for a role of glutamate and aspartate in corticostriatal neurotransmission. J Neurochem 47:98–106
Groenewegen HJ, Wright CI, Beijer AV (1996) The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res 107:485–511
Groenewegen HJ, Mulder AB, Beijer AVJ, Wright CI, Lopes Da Silva FH, Pennartz CMA (1999) Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology 27:149–164
Gundersen V, Storm-Mathisen J (2000) II. Aspartate - Neurochemical evidence for transmitter role. In: Ottersen OP, Storm-Mathisen J (eds) Handbook of chemical neuroanatomy, vol 18 - Glutamate. Elsevier/North Holland, Amsterdam, pp 45–62
Gundersen V, Chaudhry FA, Bjaalie JG, Fonnum F, Ottersen OP, Storm-Mathisen J (1998) Synaptic vesicular localization and exocytosis of L-aspartate in excitatory nerve terminals: a quantitative immunogold analysis in rat hippocampus. J Neurosci Off J Soc Neurosci 18:6059–6070
Gundersen V, Holten AT, Storm-Mathisen J (2004) GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: quantitative immunogold evidence for co-transmission. Mol Cell Neurosci 26:156–165
Husband SA, Shimizu T (2011) Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia). J Comp Neurol 519:1371–1394
Jarvis ED, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB (2005) Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6:151–159
Johnson LR, Aylward RL, Hussain Z, Totterdell S (1994) Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience 61:851–865
Kamisaki Y, Hamahashi T, Okada CM, Itoh T (1991) Clonidine inhibition of potassium-evoked release of glutamate and aspartate from rat cortical synaptosomes. Brain Res 568:193–198
Kelley AE (1999) Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology 27:198–213
Kozell LB, Meshul CK (2004) Nerve terminal glutamate immunoreactivity in the rat nucleus accumbens and ventral tegmental area after a short withdrawal from cocaine. Synapse 51:224–232
Kuenzel WJ, Masson M (1988) A stereotaxic atlas of the brian of the chick(Gallus domesticus). Johns Hopkins University Press, Baltimore
Kuenzel WJ, Medina L, Csillag A, Perkel DJ, Reiner A (2011) The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res 1424:67–101
Lada MW, Vickroy TW, Kennedy RT (1998) Evidence for neuronal origin and metabotropic receptor-mediated regulation of extracellular glutamate and aspartate in rat striatum in vivo following electrical stimulation of the prefrontal cortex. J Neurochem 70:617–625
Liu CJ, Grandes P, Matute C, Cuenod M, Streit P (1989) Glutamate-like immunoreactivity revealed in rat olfactory bulb, hippocampus and cerebellum by monoclonal antibody and sensitive staining method. Histochemistry 90:427–445
Maura G, Carbone R, Raiteri M (1989) Aspartate-releasing nerve terminals in rat striatum possess D-2 dopamine receptors mediating inhibition of release. J Pharmacol Exp Ther 251:1142–1146
McDonald AJ (1991) Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44:15–33
McDonald AJ (1996) Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol 365:367–379
Miyaji T, Echigo N, Hiasa M, Senoh S, Omote H, Moriyama Y (2008) Identification of a vesicular aspartate transporter. Proc Natl Acad Sci USA 105:11720–11724
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Morland C, Nordegen K, Larsson M, Prolo L, Reimer R, Gundersen V (2011) Aspartate: a transmitter candidate at hippocampal synapses. 8th IBRO World Congress of Neuroscience, Florence, July 14–18, Poster abstract A136
Nadler JV (2011) Aspartate release and signalling in the hippocampus. Neurochem Res 36:668–676
Nadler JV, Vaca KW, White WF, Lynch GS, Cotman CW (1976) Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature 260:538–540
Naito S, Ueda T (1985) Characterization of glutamate uptake into synaptic vesicles. J Neurochem 44:99–109
Ottersen OP, Storm-Mathisen J (1985) Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience 16:589–606
Ottersen OP, Zhang N, Walberg F (1992) Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience 46:519–534
Petrovich GD, Risold PY, Swanson LW (1996) Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 374:387–420
Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED (2004) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414
Roberts TF, Hall WS, Brauth SE (2002) Organization of the avian basal forebrain: chemical anatomy in the parrot (Melopsittacus undulatus). J Comp Neurol 454:383–408
Setlow B, Holland PC, Gallagher M (2002) Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behav Neurosci 116:267–275
Shinonaga Y, Takada M, Mizuno N (1994) Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience 58:389–397
Somogyi P, Hodgson AJ (1985) Antisera to gamma-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem Off J Histochem Soc 33:249–257
Szekely AD, Boxer MI, Stewart MG, Csillag A (1994) Connectivity of the lobus parolfactorius of the domestic chicken (Gallus domesticus): an anterograde and retrograde pathway tracing study. J Comp Neurol 348:374–393
Wiklund L, Toggenburger G, Cuenod M (1982) Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science 216:78–80
Zachar G, Wagner Z, Tabi T, Balint E, Szoko E, Csillag A (2012) Differential changes of extracellular aspartate and glutamate in the striatum of domestic chicken evoked by high potassium or distress: an in vivo microdialysis study. Neurochem Res. doi:10.1007/s11064-012-0783-4
Acknowledgments
The authors thank Mrs. Mária Szász for her devoted technical assistance in electron microscopy. Supported by OTKA K 73219 research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanics, J., Bálint, E., Milanovich, D. et al. Amygdalofugal axon terminals immunoreactive for L-aspartate or L-glutamate in the nucleus accumbens of rats and domestic chickens: a comparative electron microscopic immunocytochemical study combined with anterograde pathway tracing. Cell Tissue Res 350, 409–423 (2012). https://doi.org/10.1007/s00441-012-1494-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1494-5