Abstract
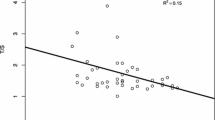
Advanced maternal age is a well-documented risk factor of chromosome 21 nondisjunction in humans, but understanding of this association at the genetic level is still limited. In particular, the state of maternal genetic age is unclear. In the present study, we estimated maternal genetic age by measuring telomere length of peripheral blood lymphocytes among age-matched mothers of children with Down syndrome (cases: N = 75) and mothers of euploid children (controls: N = 75) in an age range of 18–42 years. All blood samples were taken within 1 week of the birth of the child in both cases and controls. The telomere length estimation was performed by restriction digestion—Southern blot hybridization method. We stratified the cases on the basis of centromeric STR genotyping into maternal meiosis I (N = 48) and maternal meiosis II (N = 27) nondisjunction groups and used linear regression to compare telomere length as a function of age in the euploid, meiosis I and meiosis II groups. Our results show that all three groups have similar telomere length on average for younger mothers. As age increases, all groups show telomere loss, but that loss is largest in the meiosis II mother group and smallest in the euploid mother group with the meiosis I mother group in the middle. The regression lines for all three were statistically significantly different from each other (p < 0.001). Our results do not support the theory that younger women who have babies with Down syndrome do so because are ‘genetically older’ than their chronological age, but we provide the first evidence that older mothers who have babies with Down syndrome are “genetically older” than controls, who have euploid babies at the same age. We also show for the first time that telomere length attrition may be associated in some way with meiosis I and meiosis II nondisjunction of chromosome 21 and subsequent Down syndrome births at advanced maternal age.




Similar content being viewed by others
References
Allen EG, Freeman SB, Druschel C, Hobbs CA, O’Leary LA, Romitti PA, Royle MH, Torfs CP, Sherman SL (2009) Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet 125:41–52
Allshire RC, Dempster M, Hastie ND (1989) Human telomere contains at least three types of G-rich repeats distributed non-randomly. Nucleic Acids Res 17:4611–4627
Aviv A (2008) The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci 63:979–983
Aydos SE, Elhan AH, Tükün A (2005) Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet 272:113–116
Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, Samani NJ, Ford I (2009) Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. J Epidemiol Community Health 63:839–841
Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A (2001) Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37:381–385
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Chen JH, Hales CN, Ozanne SE (2007) DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res 35:7417–7428
Dorland M, van Montfrans JM, van Kooij RJ, Lambalk CB, te Velde ER (1998) Normal telomere lengths in young mothers of children with Down’s syndrome. Lancet 352:961–962
Eichenlaub-Ritter U, Winterscheidt U, Vogt E, Shen Y, Tinneberg HR, Sorensen R (2007) 2-Methoxyestradiol induces spindle aberrations, chromosome congression failure, and nondisjunction in mouse oocytes. Biol Reprod 76:784–793
Ghosh S, Feingold E, Dey SK (2009) Etiology of Down syndrome: evidence for consistent association among altered meiotic recombination, nondisjunction, and maternal age across populations. Am J Med Genet A 149A:1415–1420
Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP (2009) Telomere length and reproductive aging. Hum Reprod 24:1206–1211
Hassold T, Chiu D (1985) Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70:11–17
Hawley RS, Frazier JA, Rasooly R (1994) Separation anxiety: the etiology of nondisjunction in flies and people. Hum Mol Genet 3:1521–1528
Hulten M, Patel S, Jonasson J, Iwarsson E (2010) On the origin of the maternal age effect in trisomy 21 Down syndrome: the Oocyte Mosaicism Selection (OMS) model. Reproduction 139:1–9
Jeffreys CA, Burrage PS, Bickel SE (2003) A model system for increased meiotic nondisjunction in older oocyte. Curr Biol 13:498–503
Keefe DL, Liu L (2009) Telomeres and reproductive aging. Reprod Fertil 21:10–14
Keefe DL, Liu L, Marquard K (2007) Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci 64:139–143
Kline J, Levin B (1992) Trisomy and age at menopause: predicted associations given a link with rate of oocyte atresia. Paediatr Perinat Epidemiol 6:225–239
Kline J, Kinney A, Levin B, Warburton D (2000) Trisomic pregnancy and earlier age at menopause. Am J Hum Genet 67:395–404
Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M, Warburton D (2004) Trisomic pregnancy and the oocyte pool. Hum Reprod 19:1633–1643
Kline J, Kinney A, Kelly A, Reuss ML, Levin B (2005) Predictors of antral follicle count during the reproductive years. Hum Reprod 20:2179–2189
Liu L, Keefe DL (2002) Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum Reprod 17:2678–2685
Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL (2004) Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci USA 101:6496–6501
Martinez P, Siegl-Cachedenier I, Flores JM, Blasco MA (2009) MSH2 deficiency abolishes the anticancer and pro-aging activity of short telomeres. Aging Cell 8:2–17
Mendola P, Messer LC, Rappazzo K (2008) Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril 89:e81–e94
Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB (1999) Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril 71:715–718
Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, Blackburn EH, Mitchell BD, Shuldiner AR, Hsueh WC (2007) Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci USA 104:12135–121359
Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL (2008) New insight into human nondisjunction of chromosome 21 in oocyte. PloS Genet 4(3):e1000033
Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K (2005) Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod 72:107–118
Sebastián C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A (2009) Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol 183:2356–2364
Sherman SL, Allen EG, Bean LH, Freeman SB (2007) Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev 13:221–227
Susiarjo M, Hassold TJ, Freeman E, Hunt PA (2007) Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet 3:e5
Thum MY, Abdalla HI, Taylor D (2008) Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in vitro fertilization treatment. Fertil Steril 90:315–321
Van Blerkom J (2004) Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction 128:269–280
van Montfrans JM, van Hooff MH, Martens F, Lambalk CB (2002) Basal FSH, estradiol and inhibin B concentrations in women with a previous Down’s syndrome affected pregnancy. Hum Reprod 17:44–47
Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P (1998) The meiotic competence of in vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod 13:154–160
Warburton D (2005) Biological aging and the etiology of aneuploidy. Cytogenet Genome Res 111:266–272
Yoon PW, Freeman SB, Sherman SL, Taft LF, Gu Y, Pettay D, Flanders WD, Khoury MJ, Hassold TJ (1996) Advanced maternal age and the risk of Down syndrome characterized by meiotic stage of chromosomal error: a population-based study. Am J Hum Genet 58:628–633
Zwick ME, Cutler DJ, Langley CH (1999) Classic Weinstein: tetrad analysis, genetic variation and achiasmate segregation in Drosophila and humans. Genetics 152:1615–1629
Acknowledgments
We would like to thank the families participated in the study and the professionals who helped us in collection of blood samples. The project was funded by University Grants Commission (UGC), New Delhi, India; Sanctioned No. F-3-111/2001 (SR-II).
Conflict of interest statement
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, S., Feingold, E., Chakraborty, S. et al. Telomere length is associated with types of chromosome 21 nondisjunction: a new insight into the maternal age effect on Down syndrome birth. Hum Genet 127, 403–409 (2010). https://doi.org/10.1007/s00439-009-0785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0785-8