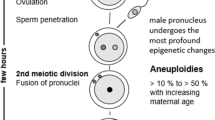
Abstract
We examined the association between maternal age and chromosome 21 nondisjunction by origin of the meiotic error. We analyzed data from two population-based, case–control studies: Atlanta Down Syndrome Project (1989–1999) and National Down Syndrome Project (2001–2004). Cases were live born infants with trisomy 21 and controls were infants without trisomy 21 delivered in the same geographical regions. We enrolled 1,215 of 1,881 eligible case families and 1,375 of 2,293 controls. We report four primary findings. First, the significant association between advanced maternal age and chromosome 21 nondisjunction was restricted to meiotic errors in the egg; the association was not observed in sperm or in post-zygotic mitotic errors. Second, advanced maternal age was significantly associated with both meiosis I (MI) and meiosis II (MII). For example, compared to mothers of controls, mothers of infants with trisomy 21 due to MI nondisjunction were 8.5 times more likely to be ≥40 years old than 20–24 years old at the birth of the index case (95% CI = 5.6–12.9). Where nondisjunction occurred in MII, mothers were 15.1 times more likely to be ≥40 years (95% CI = 8.4–27.3). Third, the ratio of MI to MII errors differed by maternal age. The ratio was lower among women <19 years of age and those ≥40 years (2.1, 2.3, respectively) and higher in the middle age group (3.6). Lastly, we found no effect of grand-maternal age on the risk for maternal nondisjunction. This study emphasizes the complex association between advanced maternal age and nondisjunction of chromosome 21 during oogenesis.


Similar content being viewed by others
References
Aagesen L, Grinsted J, Mikkelsen M (1984) Advanced grandmaternal age on the mother’s side—a risk of giving rise to trisomy 21. Ann Hum Genet 48:297–301
Antonarakis SE, Avramopoulos D, Blouin JL, Talbot CC Jr, Schinzel AA (1993) Mitotic errors in somatic cells cause trisomy 21 in about 4.5% of cases and are not associated with advanced maternal age. Nature Genet 3:146–150
Antonarakis SE, Petersen MB, McInnis MG, Adelsberger PA, Schinzel AA, Binkert F, Pangalos C, Raoul O, Slaugenhaupt SA, Hafez M (1992) The meiotic stage of nondisjunction in trisomy 21: determination by using DNA polymorphisms. Am J Hum Gen 50:544–550
Battaglia DE, Goodwin P, Klein NA, Soules MR (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Human Reprod 11:2217–2222
Book JA, Fraccaro M, Lindsten J (1959) Cytogenetical observations in Mongolism. Acta Paediatr 48:453–468
Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, Devine O, Petrini J, Ramadhani TA, Hobbs CA, Kirby RS (2006) National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res A Clin Mol Teratol 76:747–756
Carothers AD, Castilla EE, Dutra MG, Hook EB (2001) Search for ethnic, geographic, and other factors in the epidemiology of Down syndrome in South America: analysis of data from the ECLAMC project, 1967–1997. Am J Med Genet 103:149–156
Correa-Villasenor A, Cragan J, Kucik J, O’Leary L, Siffel C, Williams L (2003) The metropolitan Atlanta congenital defects program: 35 years of birth defects surveillance at the centers for disease control and prevention. Birth Defects Res A Clin Mol Teratol 67:617–624
de Bruin JP, Dorland M, Spek ER, Posthuma G, van Heaften M, Looman CW, Te Velde ER (2004) Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod 70:419–424
Eichenlaub-Ritter U, Boll I (1989) Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet Cell Genet 52:170–176
Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R (2004) Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online 8:45–58
Ford CE, Jones KW, Miller OJ, Mittwoch U, Penrose LS, Ridler M, Shapiro A (1959) The chromosomes in a patient showing both Mongolism and the Klinefelter syndrome. Lancet 1:709–710
Freeman SB, Allen EG, Oxford-Wright CL, Tinker SW, Druschel C, Hobbs CA, O’Leary LA, Romitti PA, Royle MH, Torfs CP, Sherman SL (2007) The National Down Syndrome Project: design and implementation. Public Health Rep 122:62–72
Freeman SB, Yang Q, Allran K, Taft LF, Sherman SL (2000) Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am J Hum Genet 66:1680–1683
Gaulden ME (1992) Maternal age effect: the enigma of Down syndrome and other trisomic conditions. Mutat Res 296:69–88
Gomez D, Solsona E, Guitart M, Baena N, Gabau E, Egozcue J, Caballin MR (2000) Origin of trisomy 21 in Down syndrome cases from a Spanish population registry. Ann Genet 43:23–28
Greenberg RC (1963) Two factors influencing the births of Mongols to younger mothers. Med Off 109:62–64
Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS (2004) Age-associated alteration of gene expression patterns in mouse oocytes. Human Mol Genet 13:2263–2278
Hassold T, Chiu D (1985) Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70:11–17
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev Genet 2:280–291
Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA (2002) Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod 17:1171–1180
Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA (2005) SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 37:1351–1355
Jacobs PA, Baikie AG, Court Brown WM, Strong JA (1959) The somatic chromosomes in Mongolism. Lancet 1:710
Lamb NE, Freeman SB, Savage-Austin A, Pettay D, Taft L, Hersey J, Gu Y, Shen J, Saker D, May KM, Avramopoulos D, Petersen MB, Hallberg A, Mikkelsen M, Hassold TJ, Sherman SL (1996) Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nature Genet 14:400–405
Lamb NE, Yu K, Shaffer J, Feingold E, Sherman SL (2005) Association between maternal age and meiotic recombination for trisomy 21. Am J Hum Genet 76:91–99
Lejeune J (1959) Le Mongolism. Premier exemple d’aberration autosomique humaine. Annals of Genetics 1:41–49
LeMaire-Adkins R, Radke K, Hunt PA (1997) Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol 139:1611–1619
Malini SS, Ramachandra NB (2006) Influence of advanced age of maternal grandmothers on Down syndrome. BMC Med Genet 7:4
Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML (2005) Births: final data for 2003. Natl Vital Stat Rep 54(2):1–116
Mikkelsen M, Hallberg A, Poulsen H, Frantzen M, Hansen J, Petersen MB (1995) Epidemiology study of Down’s syndrome in Denmark, including family studies of chromosomes and DNA markers. Develop Brain Dysfunct 8:4–12
Muller F, Rebiffe M, Taillandier A, Oury JF, Mornet E (2000) Parental origin of the extra chromosome in prenatally diagnosed fetal trisomy 21. Hum Genet 106:340–344
Mutton D, Alberman E, Hook EB (1996) Cytogenetic and epidemiological findings in Down syndrome, England and Wales 1989 to 1993. National Down syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J Med Genet 33:387–394
Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL (2008) New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet 4:e1000033
Pan H, Ma P, Zhu W, Schultz RM (2008) Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 316:397–407
Papp Z, Varadi E, Szabo Z (1977) Grandmaternal age at birth of parents of children with trisomy 21. Hum Genet 39:221–224
Pellestor F, Anahory T, Hamamah S (2005) Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res 111:206–212
Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J (2002) Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum Reprod 17:2134–2145
Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J (2003) Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet 112:195–203
Penrose LS (1933) The relative effects of paternal and maternal age in Mongolism. J Genet 27:219–224
Penrose LS (1934) The relative aetiological importance of birth order and maternal age in Mongolism. Proc R Soc B Biol Sci 115:431–450
Penrose LS (1964) Genetical aspects of mental deficiency. Proceedings of the international Copenhagen congress on the scientific study of mental retardation, pp 165–172
Petersen MB, Antonarakis SE, Hassold TJ, Freeman SB, Sherman SL, Avramopoulos D, Mikkelsen M (1993) Paternal nondisjunction in trisomy 21: excess of male patients. Human Mol Genet 2:1691–1695
Richards BW (1970) Observations on mosaic parents of mongol propositi. J Ment Defic Res 14:342–346
Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ (2000) Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod 15(Suppl 2):160–172
Sherman SL, Freeman SB, Allen EG, Lamb NE (2005) Risk factors for nondisjunction of trisomy 21. Cytogenet Genome Res 111:273–280
Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J (2007) Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online 14:700–708
Steuerwald NM, Steuerwald MD, Mailhes JB (2005) Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod 11:623–630
Stoller A, Collmann RD (1969) Grandmaternal age at birth of mothers of children with Down’s syndrome (ONGOLISM). J Ment Defic Res 13:201–205
van Montfrans JM, van Hooff MH, Martens F, Lambalk CB (2002) Basal FSH, estradiol and inhibin B concentrations in women with a previous Down’s syndrome affected pregnancy. Human Reprod 17:44–47
Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U (2008) Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 651:14–29
Warburton D (2005) Biological aging and the etiology of aneuploidy. Cytogenet Genome Res 111:266–272
Yang Q, Sherman SL, Hassold TJ, Allran K, Taft L, Pettay D, Khoury MJ, Erickson JD, Freeman SB (1999) Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case–control study. Genet Med 1:80–88
Yoon PW, Freeman SB, Sherman SL, Taft LF, Gu Y, Pettay D, Flanders WD, Khoury MJ, Hassold TJ (1996) Advanced maternal age and the risk of Down syndrome characterized by the meiotic stage of chromosomal error: a population-based study. Am J Hum Genet 58:628–633
Yusuf RZ, Naeem R (2004) Cytogenetic abnormalities in products of conception: a relationship revisited. Am J Reprod Immunol 52:88–96
Acknowledgments
We gratefully acknowledge the many families nationwide whose participation has made this study possible. In addition, we want to thank all personnel at each NDSP site and their associated birth surveillance teams who made this project a success. Lastly, we would like to thank Larry Edmonds and Dr. Paula Yoon who shared their experience with the National Birth Defects Prevention Study. Their help was invaluable. This work was supported by NIH R01 HD38979 and by the technical assistance of the General Clinical Research Center at Emory University (NIH/NCRR M01 RR00039).
Author information
Authors and Affiliations
Corresponding author
Additional information
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Rights and permissions
About this article
Cite this article
Allen, E.G., Freeman, S.B., Druschel, C. et al. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet 125, 41–52 (2009). https://doi.org/10.1007/s00439-008-0603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-008-0603-8