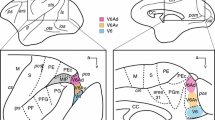
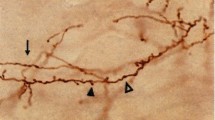
Abstract
Individual axons that form the hyperdirect pathway in Macaca fascicularis were visualized following microiontophoretic injections of biotinylated dextran amine in layer V of the primary motor cortex (M1). Twenty-eight singly labeled axons were reconstructed in 3D from serial sections. The M1 innervation of the subthalamic nucleus (STN) arises essentially from collaterals of long-ranged corticofugal axons en route to lower brainstem regions. Typically, after leaving M1, these large caliber axons (2–3 µm) enter the internal capsule and travel between caudate nucleus and putamen without providing any collateral to the striatum. More ventrally, they emit a thin collateral (0.5–1.5 µm) that runs lateromedially within the dorsal region of the STN, providing boutons en passant in the sensorimotor territory of the nucleus. In some cases, the medial tip of the collateral enters the lenticular fasciculus dorsally and yields a few beaded axonal branches in the zona incerta. In other cases, the collateral runs caudally and innervates the ventrolateral region of the red nucleus where large axon varicosities (up to 1.7 µm in diameter) are observed, many displaying perisomatic arrangements. Our ultrastructural analysis reveals a high synaptic incidence (141%) of cortical VGluT1-immunoreactive axon varicosities on distal dendrites of STN neurons, and on various afferent axons. Our single-axon reconstructions demonstrate that the so-called hyperdirect pathway derives essentially from collaterals of long-ranged corticofugal axons that are rarely exclusively devoted to the STN, as they also innervate the red nucleus and/or the zona incerta.






Similar content being viewed by others
Abbreviations
- ABC:
-
Avidine–biotin complex
- AS:
-
Associative
- av:
-
Axon varicosity
- BDA:
-
Biotinylated dextran amine
- BG:
-
Basal ganglia
- bs:
-
Brainstem
- Cd:
-
Caudate nucleus
- d:
-
Dendrite
- DAB:
-
Diaminobenzidine
- DBS:
-
Deep brain stimulation
- GABA:
-
Gamma-aminobutyric acid
- Glu:
-
Glutamate
- GP:
-
Globus pallidus
- GPe:
-
External segment of the globus pallidus (external pallidum)
- GPi:
-
Internal segment of the globus pallidus (internal pallidum)
- H1 :
-
Forel’s field H1 (thalamic fasciculus)
- H2 :
-
Forel’s field H2 (lenticular fasciculus)
- ic:
-
Internal capsule
- LI:
-
Limbic
- M1:
-
Primary motor cortex
- PB:
-
Phosphate buffer
- PBS:
-
Phosphate buffer saline
- PD:
-
Parkinson’s disease
- PFA:
-
Paraformaldehyde
- Put:
-
Putamen
- RN:
-
Red nucleus
- rt:
-
Reticular thalamic nucleus
- SM:
-
Sensorimotor
- SN:
-
Substantia nigra
- SNr:
-
Substantia nigra pars reticulata
- SPN:
-
Superior pontine nucleus
- STN:
-
Subthalamic nucleus
- Th:
-
Thalamus
References
Afsharpour S (1985) Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol 236(1):14–28. https://doi.org/10.1002/cne.902360103
Akram H, Sotiropoulos SN, Jbabdi S, Georgiev D, Mahlknecht P, Hyam J, Foltynie T, Limousin P, De Vita E, Jahanshahi M (2017) Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. NeuroImage 158:332–345
Anderson RW, Farokhniaee A, Gunalan K, Howell B, McIntyre CC (2018) Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimul. https://doi.org/10.1016/j.brs.2018.05.008
Beaudet A, Sotelo C (1981) Synaptic remodeling of serotonin axon terminals in rat agranular cerebellum. Brain Res 206(2):305–329
Bevan MD, Francis CM, Bolam JP (1995) The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol 361(3):491–511. https://doi.org/10.1002/cne.903610312
Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA (1990) Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res 513(1):43–59
Chu H-Y, McIver EL, Kovaleski RF, Atherton JF, Bevan MD (2017) Loss of hyperdirect pathway cortico-subthalamic inputs following degeneration of midbrain dopamine neurons. Neuron 95(6):1306–1318 (e1305)
Clarke NP, Bolam JP (1998) Distribution of glutamate receptor subunits at neurochemically characterized synapses in the entopeduncular nucleus and subthalamic nucleus of the rat. J Comp Neurol 397(3):403–420
DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64(1):20–24
DeLong MR, Crutcher MD, Georgopoulos AP (1985) Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol 53(2):530–543. https://doi.org/10.1152/jn.1985.53.2.530
Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, Dauguet J, Lefaucheur JP, Dolle F, Conde F, Bottlaender M, Peschanski M, Keravel Y, Hantraye P, Palfi S (2004) Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron 44(5):769–778. https://doi.org/10.1016/j.neuron.2004.11.023
Feger J, Bevan M, Crossman AR (1994) The projections from the parafascicular thalamic nucleus to the subthalamic nucleus and the striatum arise from separate neuronal populations: a comparison with the corticostriatal and corticosubthalamic efferents in a retrograde fluorescent double-labelling study. Neuroscience 60(1):125–132
Fujimoto K, Kita H (1993) Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res 609(1–2):185–192
Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T (2006) Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. Eur J Neurosci 24(10):2813–2824. https://doi.org/10.1111/j.1460-9568.2006.05177.x
Gagnon D, Parent M (2014) Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS One 9(2):e87709
Galvan A, Wichmann T (2008) Pathophysiology of parkinsonism. Clin Neurophysiol 119(7):1459–1474
Georgopoulos AP, DeLong MR, Crutcher MD (1983) Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci 3(8):1586–1598
Gerfen CR, Bolam JP (2017) The neuroanatomical organization of the basal ganglia. In: Steiner H, Tseng KY (eds) Handbook of basal ganglia structure and function, 2nd edn. Elsevier, Amsterdam, pp 3–32
Giuffrida R, Volsi GL, Maugeri G, Perciavalle V (1985) Influences of pyramidal tract on the subthalamic nucleus in the cat. Neurosci Lett 54(2):231–235
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (2009) Optical deconstruction of parkinsonian neural circuitry. Science 324(5925):354–359. https://doi.org/10.1126/science.1167093
Hammond C, Yelnik J (1983) Intracellular labelling of rat subthalamic neurones with horseradish peroxidase: computer analysis of dendrites and characterization of axon arborization. Neuroscience 8(4):781–790
Hartmann-von Monakow K, Akert K, Künzle H (1978) Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res 33(3–4):395–403
Haynes WI, Haber SN (2013) The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci 33(11):4804–4814
Hicks TP, Onodera S (2012) The mammalian red nucleus and its role in motor systems, including the emergence of bipedalism and language. Prog Neurobiol 96(2):165–175
Huerta MF, Kaas JH (1990) Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293(2):299–330
Huerta MF, Krubitzer LA, Kaas JH (1986) Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. Subcortical connections. J Comp Neurol 253(4):415–439. https://doi.org/10.1002/cne.902530402
Iwahori N (1978) A Golgi study on the subthalamic nucleus of the cat. J Comp Neurol 182(3):383–397. https://doi.org/10.1002/cne.901820303
Iwamuro H, Tachibana Y, Ugawa Y, Saito N, Nambu A (2017) Information processing from the motor cortices to the subthalamic nucleus and globus pallidus and their somatotopic organizations revealed electrophysiologically in monkeys. Eur J Neurosci 46:2684–2701
Jurgens U (1984) The efferent and afferent connections of the supplementary motor area. Brain Res 300(1):63–81
Kita H, Kita T (2011) Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J Neurosci 31(28):10311–10322
Kita T, Kita H (2012) The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 32(17):5990–5999
Kitai S, Deniau J (1981) Cortical inputs to the subthalamus: intracellular analysis. Brain Res 214(2):411–415
Kuwajima M, Hall RA, Aiba A, Smith Y (2004) Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol 474(4):589–602. https://doi.org/10.1002/cne.20158
Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y (2007) Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci 27(23):6249–6260. https://doi.org/10.1523/JNEUROSCI.3819-06.2007
Kwan H, MacKay W, Murphy J, Wong Y (1978) Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41(5):1120–1131
Lemon RN (2016) Cortical projections to the red nucleus and the brain stem in the rhesus monkey. Brain Res 1645:28–30
Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D (2007) Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98(6):3525–3537
Mathai A, Ma Y, Paré J-F, Villalba RM, Wichmann T, Smith Y (2015) Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Brain 138(4):946–962
Miller J (2017) The reticular thalamic nucleus: revealing a novel phenotype of neurons and describing changes in a rat model of Parkinson’s disease. University of Otago, Dunedin
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425
Mitrofanis J (2005) Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience 130(1):1–15
Nambu A, Takada M, Inase M, Tokuno H (1996) Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 16(8):2671–2683
Nambu A, Tokuno H, Inase M, Takada M (1997) Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett 239(1):13–16
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci Res 43(2):111–117
Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M (2008) Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord 23:(S3)
Parent A (1990) Extrinsic connections of the basal ganglia. Trends Neurosci 13(7):254–258
Parent A, Hazrati L-N (1995) Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev 20(1):128–154
Parent M, Parent A (2005) Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol 481(1):127–144. https://doi.org/10.1002/cne.20348
Parent M, Parent A (2006) Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol 496(2):202–213
Parent M, Parent A (2016) The primate basal ganglia connectome as revealed by single-axon tracing. In: Rockland KS (ed) Axons and brain architecture. Elsevier, Amsterdam, pp 27–46
Parent M, Lévesque M, Parent A (2001) Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol 439(2):162–175
Pinault D (2004) The thalamic reticular nucleus: structure, function and concept. Brain Res Rev 46(1):1–31
Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y (2006) Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol 499(2):231–243. https://doi.org/10.1002/cne.21099
Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y (2008) Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci 27(7):1647–1658
Romansky KV, Usunoff KG, Ivanov DP, Galabov GP (1979) Corticosubthalamic projection in the cat: an electron microscopic study. Brain Res 163(2):319–322
Rouzaire-Dubois B, Scarnati E (1985) Bilateral corticosubthalamic nucleus projections: an electrophysiological study in rats with chronic cerebral lesions. Neuroscience 15(1):69–79
Sadikot AF, Parent A, Francois C (1992) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol 315(2):137–159. https://doi.org/10.1002/cne.903150203
Sato F, Parent M, Lévesque M, Parent A (2000) Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol 424(1):142–152
Shink E, Bevan MD, Bolam JP, Smith Y (1996) The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience 73(2):335–357
Shook BL, Schlag-Rey M, Schlag J (1991) Primate supplementary eye field. II. Comparative aspects of connections with the thalamus, corpus striatum, and related forebrain nuclei. J Comp Neurol 307(4):562–583. https://doi.org/10.1002/cne.903070405
Stanton GB, Goldberg ME, Bruce CJ (1988) Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol 271(4):473–492. https://doi.org/10.1002/cne.902710402
Steiner H, Tseng KY (2016) Handbook of basal ganglia structure and function, vol 24. Academic Press, Cambridge
Stepniewska I, Preuss TM, Kaas JH (1993) Architectionis, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol 330(2):238–271
Szabo J, Cowan W (1984) A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol 222(2):265–300
Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK (1994) Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol 348(3):351–373
Villalba RM, Smith Y (2011) Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated parkinsonian monkeys. J Comp Neurol 519(5):989–1005
Wichmann T, DeLong MR (2016) Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics 13(2):264–283
Wichmann T, Bergman H, DeLong MR (2017) Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research. J Neural Transm 125:1–12
Wong-Riley M (1979) Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171(1):11–28
Woolsey CN (1958) Organization of somatic sensory and motor areas of the cerebral cortex. In: Harlow HF, Woolsey CN (eds) Biological and biochemical behaviour. University of Wisconsin Press, Madison, pp 63–81
Yelnik J, Percheron G (1979) Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience 4(11):1717–1743
Funding
The study was supported by research grants from the Canadian Institutes of Health Research (CIHR MOP-153068) and the Natural Sciences and Engineering Research Council of Canada (NSERC 2018-06264 and 2018-522690) to M.P. who also benefited from a Junior II career award from the Fonds de Recherche du Québec-Santé (FRQ-S). D.C. was the recipient of MSc fellowship from FRQ-S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures were approved by the Comité de Protection des Animaux de l’Université Laval, in accordance with the Canadian Council on Animal Care’s Guide to the Care and Use of Experimental Animals (Ed2). Maximum efforts were made to minimize the number of animals used.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Coudé, D., Parent, A. & Parent, M. Single-axon tracing of the corticosubthalamic hyperdirect pathway in primates. Brain Struct Funct 223, 3959–3973 (2018). https://doi.org/10.1007/s00429-018-1726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1726-x