Abstract
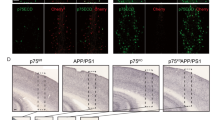
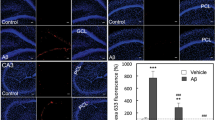
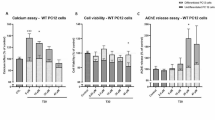
A fascinating yet perhaps overlooked trait of the p75 neurotrophin receptor (p75NTR) is its ability to bind ligands with no obvious neurotrophic function. Using cultured basal forebrain (BF) neurons, this study demonstrates selective internalization of amyloid β (Aβ) 1–42 in conjunction with p75NTR (labelled with IgG192-Cy3) by cholinergic cells. Active under resting conditions, this process was enhanced by high K+ stimulation and was insensitive to inhibitors of regulated synaptic activity—tetrodotoxin or botulinum neurotoxins (BoNT type/A and/B). Blockade of sarco-endoplasmic reticulum (SERCA) Ca2+ ATPase with thapsigargin and CPA or chelation of Ca2+ with EGTA-AM strongly suppressed the endocytosis of p75NTR, implicating the role of ER released Ca2+. The uptake of IgG192-Cy3 was also reduced by T-type Ca2+ channel blocker mibefradil but not Cd2+, an indiscriminate blocker of high voltage-activated Ca2+ currents. A strong co-localization of IgG192-Cy3 with late endosome (Rab7) or lysosome (Lamp1) qualifier proteins suggest these compartments as the primary destination for internalized IgG192 and Aβ. Selective uptake and labeling of BF cholinergic cells with IgG192-Cy3 injected into the prefrontal cortex was verified also in vivo. The significance of these findings in relation to Aβ clearance in the cerebral cortex and pathophysiology of Alzheimer’s disease is discussed.






Similar content being viewed by others
References
Allen SJ, Watson JJ, Dawbarn D (2011) The neurotrophins and their role in Alzheimer's disease. Curr Neuropharmacol 9(4):559–573
Almeida CG, Takahashi RH, Gouras GK (2006) Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci 26:4277–4288
Arevalo MA, Roldan PM, Chacon PJ, Rodriguez-Tebar A (2009) Amyloid beta serves as an NGF-like neurotrophic factor or acts as a NGF antagonist depending on its concentration. J Neurochem 111:1425–1433
Auld DS, Day JC, Mennicken F, Quirion R (2000) Pharmacological characterization of endogenous acetylcholine release from primary septal cultures. J Pharmacol Exp Ther 292:692–697
Baldwin AN, Shooter EM (1995) Zone mapping of the binding domain of the rat low affinity nerve growth factor receptor by the introduction of novel N-glycosylation sites. J Biol Chem 270:4594–4602
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Butowt R, von Bartheld CS (2009) Fates of neurotrophins after retrograde axonal transport: phosphorylation of p75NTR is a sorting signal for delayed degradation. J Neurosci 29:10715–10729
Buzsaki G, Gage FH (1989) The cholinergic nucleus basalis: a key structure in neocortical arousal. Exs 57:159–171
Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C (2001) Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J 20:683–693
Chakravarthy B, Menard M, Ito S, Gaudet C, Dal Pra I, Armato U, Whitfield J (2012) Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J Alzheimers Dis 30:675–684
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309
Coulson EJ, May LM, Osborne SL, Reid K, Underwood CK, Meunier FA, Bartlett PF, Sah P (2008) p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. J Neurosci 28:315–324
Coulson EJ, May LM, Sykes AM, Hamlin AS (2009) The role of the p75 neurotrophin receptor in cholinergic dysfunction in Alzheimer’s disease. Neuroscientist 15:317–323
Counts SE, Mufson EJ (2005) The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol 64:263–272
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–32053
Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER (2009) Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci 12:767–776
Dechant G, Barde YA (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5:1131–1136
Ertel SI, Ertel EA (1997) Low-voltage-activated T-type Ca2+ channels. Trends Pharmacol Sci 18:37–42
Ertel SI, Ertel EA, Clozel JP (1997) T-type Ca2+ channels and pharmacological blockade: potential pathophysiological relevance. Cardiovasc Drugs Ther 11:723–739
Etheredge JA, Murchison D, Abbott LC, Griffith WH (2007) Functional compensation by other voltage-gated Ca2+ channels in mouse basal forebrain neurons with Ca(V)2.1 mutations. Brain Res 1140:105–119
Gad H, Low P, Zotova E, Brodin L, Shupliakov O (1998) Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron 21:607–616
Geula C, Mesulam MM, Saroff DM, Wu CK (1998) Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer disease. J Neuropathol Exp Neurol 57:63–75
Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ (2006) Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J Neurochem 97:475–487
Griffith WH, Taylor L, Davis MJ (1994) Whole-cell and single-channel calcium currents in guinea pig basal forebrain neurons. J Neurophysiol 71:2359–2376
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hartig W, Seeger J, Naumann T, Brauer K, Bruckner G (1998) Selective in vivo fluorescence labelling of cholinergic neurons containing p75(NTR) in the rat basal forebrain. Brain Res 808:155–165
Hartmann M, Heumann R, Lessmann V (2001) Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J 20:5887–5897
Hasselmo ME, Giocomo LM (2006) Cholinergic modulation of cortical function. J Mol Neurosci 30:133–135
Hefti F, Weiner WJ (1986) Nerve growth factor and Alzheimer’s disease. Ann Neurol 20:275–281
Herreros J, Ng T, Schiavo G (2001) Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol Biol Cell 12:2947–2960
Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, Mobley WC (1999) Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem 274:36707–36714
Huerta PT, Lisman JE (1995) Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15:1053–1063
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500
Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL (2004) Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117:4837–4848
Kacza J, Grosche J, Seeger J, Brauer K, Bruckner G, Hartig W (2000) Laser scanning and electron microscopic evidence for rapid and specific in vivo labelling of cholinergic neurons in the rat basal forebrain with fluorochromated antibodies. Brain Res 867:232–238
Lacy DB, Stevens RC (1997) Recombinant expression and purification of the botulinum neurotoxin type A translocation domain. Protein Expr Purif 11:195–200
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509
Lah JJ, Levey AI (2000) Endogenous presenilin-1 targets to endocytic rather than biosynthetic compartments. Mol Cell Neurosci 16:111–126
Lalli G, Schiavo G (2002) Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR. J Cell Biol 156:233–239
Langui D, Girardot N, El Hachimi KH, Allinquant B, Blanchard V, Pradier L, Duyckaerts C (2004) Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice. Am J Pathol 165:1465–1477
Lawrence JJ (2008) Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci 31:317–327
Lawrence JJ, Grinspan ZM, Statland JM, McBain CJ (2006) Muscarinic receptor activation tunes mouse stratum oriens interneurones to amplify spike reliability. J Physiol 571:555–562
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071
McKinney M, Jacksonville MC (2005) Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol 70:1115–1124
Mesulam MM (1990) Human brain cholinergic pathways. Prog Brain Res 84:231–241
Mesulam M (2004) The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn Mem 11:43–49
Mudd LM, Torres J, Lopez TF, Montague J (1998) Effects of growth factors and estrogen on the development of septal cholinergic neurons from the rat. Brain Res Bull 45:137–142
Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST (2003) Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat 26(4):233–242
Mullins C, Bonifacino JS (2001) The molecular machinery for lysosome biogenesis. BioEssays 23:333–343
Murchison D, Griffith WH (1995) Low-voltage activated calcium currents increase in basal forebrain neurons from aged rats. J Neurophysiol 74:876–887
Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA (2002) Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci 22:2409–2418
Nazer B, Hong S, Selkoe DJ (2008) LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis 30:94–102
Nitsch RM, Slack BE, Wurtman RJ, Growdon JH (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258:304–307
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120:4081–4091
O’Leary VB, Ovsepian SV, Raghunath A, Huo Q, Lawrence GW, Smith L, Dolly JO (2011) Innocuous full-length botulinum neurotoxin targets and promotes the expression of lentiviral vectors in central and autonomic neurons. Gene Ther 18:656–665
Ovsepian SV (2008) Differential cholinergic modulation of synaptic encoding and gain control mechanisms in rat hippocampus. Neurosci Res 61:92–98
Ovsepian SV, Dolly JO (2011) Dendritic SNAREs add a new twist to the old neuron theory. Proc Natl Acad Sci USA 108:19113–19120
Ovsepian SV, Friel DD (2012) Enhanced synaptic inhibition disrupts the efferent code of cerebellar Purkinje neurons in leaner Cav2.1 Ca2+ channel mutant mice. Cerebellum 11:666–680
Ovsepian SV, Anwyl R, Rowan MJ (2004) Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci 20:1267–1275
Ovsepian SV, Dolly JO, Zaborszky L (2012) Intrinsic voltage dynamics govern the diversity of spontaneous firing profiles in basal forebrain noncholinergic neurons. J Neurophysiol 108:406–418
Patel AN, Jhamandas JH (2012) Neuronal receptors as targets for the action of amyloid-beta protein (Abeta) in the brain. Expert Rev Mol Med 14:e2
Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP (2007) Internalization of beta-amyloid peptide by primary neurons in the absence of apolipoprotein E. J Biol Chem 282:35722–35732
Salinas S, Schiavo G, Kremer EJ (2010) A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 8:645–655
Shupliakov O (2009) The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release. Neuroscience 158:204–210
Smith LA (1998) Development of recombinant vaccines for botulinum neurotoxin. Toxicon 36:1539–1548
Sotthibundhu A, Sykes AM, Fox B, Underwood CK, Thangnipon W, Coulson EJ (2008) Beta-amyloid(1-42) induces neuronal death through the p75 neurotrophin receptor. J Neurosci 28:3941–3946
Swaab DF (1991) Brain aging and Alzheimer’s disease, “wear and tear” versus “use it or lose it”. Neurobiol Aging 12:317–324
Takahashi RH, Nam EE, Edgar M, Gouras GK (2002a) Alzheimer beta-amyloid peptides: normal and abnormal localization. Histol Histopathol 17:239–246
Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK (2002b) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161:1869–1879
Taniuchi M, Johnson EM Jr (1985) Characterization of the binding properties and retrograde axonal transport of a monoclonal antibody directed against the rat nerve growth factor receptor. J Cell Biol 101:1100–1106
Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP (1988) Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci 11:431–438
Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135:913–924
Van der Zee CE, Ross GM, Riopelle RJ, Hagg T (1996) Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science 274:1729–1732
Wang YJ, Wang X, Lu JJ, Li QX, Gao CY, Liu XH, Sun Y, Yang M, Lim Y, Evin G, Zhong JH, Masters C, Zhou XF (2011) p75NTR regulates Abeta deposition by increasing Abeta production but inhibiting Abeta aggregation with its extracellular domain. J Neurosci 31:2292–2304
Wayman CP, McFadzean I, Gibson A, Tucker JF (1996) Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol 117:566–572
Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, Gilchrest BA (1997) Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J Clin Invest 100:2333–2340
Yaar M, Zhai S, Fine RE, Eisenhauer PB, Arble BL, Stewart KB, Gilchrest BA (2002) Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem 277:7720–7725
Yan Z, Feng J (2004) Alzheimer’s disease: interactions between cholinergic functions and beta-amyloid. Curr Alzheimer Res 1:241–248
Zaborszky L (2002) The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res 136:359–372
Zeng F, Lu JJ, Zhou XF, Wang YJ (2011) Roles of p75NTR in the pathogenesis of Alzheimer’s disease: a novel therapeutic target. Biochem Pharmacol 82:1500–1509
Acknowledgments
This work was supported by the Program for Research in Third Level Institutions Cycle 4 grant from the Irish Higher Educational Authority for the Neuroscience section of ‘Targeted-driven therapeutics and theranostics’ (to Saak V. Ovsepian and J. Oliver Dolly), NIH/NINDS NS023945 grant (to Laszlo Zaborszky) and Deutsche Forschungsgemeinschaft within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy) (Jochen Herms). Authors thank Liam Ryan for expressing and purifying BoNT/A and/B.
Author information
Authors and Affiliations
Corresponding authors
Additional information
S. V. Ovsepian and I. Antyborzec contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ovsepian, S.V., Antyborzec, I., O’Leary, V.B. et al. Neurotrophin receptor p75 mediates the uptake of the amyloid beta (Aβ) peptide, guiding it to lysosomes for degradation in basal forebrain cholinergic neurons. Brain Struct Funct 219, 1527–1541 (2014). https://doi.org/10.1007/s00429-013-0583-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0583-x